A single priming dose of tremelimumab and durvalumab, followed by monthly durvalumab, showed clinical activity in a predominantly second-line advanced hepatocellular carcinoma population, in a study reported at the ESMO World Congress on Gastrointestinal Cancer Virtual 2020.1
In a study of 332 patients, the overall response rate was 24%, the median duration of response was not reached, and the median overall survival was 18.7 months, according to Bruno Sangro, MD, PhD, Director of the Liver Unit at Clinica Universidad de Navarra and Professor of Medicine at the University of Navarra, Pamplona, Spain.
“The survival curves separated nicely from the beginning for the cohorts receiving the higher doses of tremelimumab,” said Dr. Sangro, who presented the findings on behalf of the study’s first author, R. Katie Kelley, MD, of the University of California, San Francisco.
“The survival curves separated nicely from the beginning for the cohorts receiving the higher doses of tremelimumab.”— Bruno Sangro, MD, PhD
Tweet this quote
Rationale for Priming Approach
Immune checkpoint inhibition with anti–PD-L1 and anti–CTLA-4 antibodies has produced durable tumor responses in subsets of patients with advanced hepatocellular carcinoma. There is a strong rationale, therefore, for attempting to enhance antitumor activity by combining the anti–CTLA-4 antibody tremelimumab with the anti–PD-L1 agent durvalumab, which target two different and complementary immune mechanisms.
A potential issue with combining these two classes of immunotherapies has been toxicity, but the investigators have attempted to ameliorate it through a novel dosing approach. In patients with non–small cell lung cancer or melanoma treated with tremelimumab plus durvalumab, “priming” with the initial dose caused a “proliferative burst” of peripheral T cells not seen with subsequent doses of these agents. The current study asked whether a similar effect might be achieved in advanced hepatocellular carcinoma.
“Thus, the question raised was whether a single priming dose of tremelimumab with durvalumab would improve immune-mediated clinical activity in patients with hepatocellular carcinoma while minimizing toxicity,” Dr. Sangro explained.
Study Design
The three-part study included 332 patients with unresectable hepatocellular carcinoma and Child-Pugh A liver function whose disease progressed on or they were intolerant to or refused prior sorafenib. Patients were randomly assigned to the following treatment arms:
- Tremelimumab at 300 mg (T300) × 1 dose plus durvalumab at 1,500 mg every 4 weeks (n = 75)
- Durvalumab at 1,500 mg every 4 weeks (durvalumab monotherapy; n = 104)
- Tremelimumab at 750 mg (T750) every 4 weeks x 7 doses, then every 12 weeks thereafter (tremelimumab monotherapy; n = 69)
- Tremelimumab at 75 mg (T75) x 4 doses plus durvalumab at 1,500 mg every 4 weeks (n = 84).
Approximately 40% of patients were recruited from Asian countries, excluding Japan. Approximately 30% were naive to sorafenib, and a significant number had poor-prognosis factors such as extrahepatic spread (> 50%) or high levels of alpha-fetoprotein (approximately 40%).
Priming Dose Led to Longest Overall Survival
The median overall survival was longest for T300 plus durvalumab at 18.7 months and second longest for tremelimumab monotherapy (T750) at 15.1 months, with curves separating from the other cohorts quite early on, Dr. Sangro reported (Table 1).
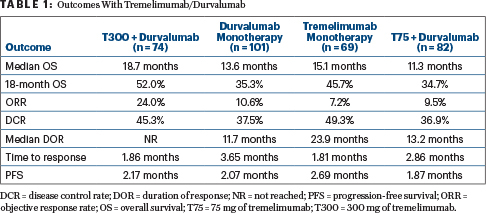
“In alignment with the survival data, the highest objective response rate (24%) was seen with T300 plus durvalumab. Responses in that arm also occurred early and were sustained over time,” he said. Progression-free survival did not appreciably differ between the arms and was less than 3 months in all.
Responders across all four cohorts tended to have a lymphocyte population composed of proliferating, Ki67-positive, CD8-positive T cells. The highest values were observed in patients responding to T300 plus durvalumab. This regimen also had the best benefit/risk profile, with just 25% of patients requiring steroids for adverse events and 10.8% discontinuing treatment because of treatment-related adverse events.
Treatment-related serious adverse events were rare, observed in 16% of the T300-plus-durvalumab arm, 11% of the durvalumab-monotherapy arm, 25% of the tremelimumab-monotherapy (T750) arm, and 15% of the T75-plus-durvalumab arm. Those requiring steroids or discontinuation of treatment were more commonly receiving the higher doses of tremelimumab. Immune-mediated adverse events requiring steroids were also uncommon, and colitis and pneumonitis were infrequent.
T300 plus durvalumab and the durvalumab monotherapy regimen are being compared with sorafenib in an ongoing phase III trial.
DISCLOSURE: Dr. Sangro has served as an advisor to or on the speakers bureaus of Adaptimmune, AstraZeneca, Bayer, Bristol Myers Squibb, BTG, Onxeo, H3Biomedicine, Ipsen, Lilly, Merck, Roche/Genentech, and Sirtex Medical and has received research funding from Bristol Myers Squibb, Onxeo, and Sirtex Medical.
REFERENCE
1. Kelley RK, Kudo M, Harris W, et al: The novel regimen of tremelimumab in combination with durvalumab provides a favorable safety profile and clinical activity for patients with advanced hepatocellular carcinoma. ESMO World Congress on Gastrointestinal Cancer 2020 Virtual. Abstract O-6.

