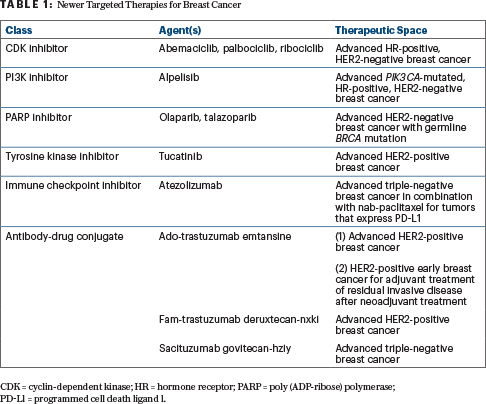
Novel targeted therapies have increased the likelihood of cure and prolonged survival in many patients with advanced breast cancer (Table 1), but these new agents also carry toxicity profiles that vary greatly from those of traditional chemotherapy. During the ASCO20 Virtual Education Program, Alexandra Thomas, MD, FACP, an oncologist at Wake Forest Baptist Medical Center Comprehensive Cancer Center, in Winston-Salem, North Carolina, discussed ongoing efforts to balance the promise of therapeutic efficacy and treatment-related adverse events.1
CDK Inhibitors: Palbociclib, Ribociclib, and Abemaciclib
As Dr. Thomas reported, cyclin-dependent kinase (CDK) inhibitors are now standard of care in the first-line setting for advanced hormone receptor–positive breast cancer, with multiple studies having demonstrated both progression-free and overall survival benefit with the addition of CDK inhibition to antiestrogen therapy compared with antiestrogen therapy or chemotherapy alone.2 Because none of the three U.S. Food and Drug Administration (FDA)-approved agents in this class (palbociclib, ribociclib, and abemaciclib) have shown superiority over the other, however, specific toxicity profiles must be considered when selecting a CDK inhibitor for an individual patient.
One notable and frequent toxicity is neutropenia, said Dr. Thomas, who recommended regular, upfront monitoring that can be de-escalated over time. Because CDK inhibition is infrequently associated with febrile neutropenia, however, routine use of granulocyte colony-stimulating factor is not advised. The frequency of grade 3 and 4 neutropenia also decreases with extended therapy, suggesting cumulative toxicity is unlikely, said Dr. Thomas.
Abemaciclib is also associated with diarrhea, and ribociclib is associated with QTc prolongation and liver abnormalities. Dose reductions and monitoring are recommended for these side effects, said Dr. Thomas, who noted that dose reductions per guidelines are generally effective and do not appear to impact progression-free survival.
PI3K Inhibitor: Alpelisib
FDA approval of the first PI3K inhibitor for the treatment of breast cancer was based on data from the SOLAR-1 trial, which showed a median progression-free survival of 11 months with the addition of alpelisib to fulvestrant in patients with PIK3CA-mutated tumors vs 5.7 months for fulvestrant plus placebo.3
Notable adverse events seen with alpelisib were hyperglycemia, rash, and diarrhea, said Dr. Thomas, and more than 75% of patients who received alpelisib had grade 3 or 4 toxicity. Permanent discontinuation of the agent due to adverse events was seen in 25% of patients who received alpelisib and in 4.2% of those who received placebo.
PARP Inhibitors: Olaparib and Talazoparib
Two oral poly (ADP-ribose) polymerase (PARP) inhibitors, olaparib and talazoparib, are available for the treatment of advanced breast cancer in patients with known or suspected deleterious germline BRCA mutations, and both agents have demonstrated a progression-free survival benefit compared with single-agent chemotherapy in patients previously treated with chemotherapy in the neoadjuvant, adjuvant, or metastatic setting.4,5 Although an overall survival benefit was not demonstrated, said Dr. Thomas, follow-up was limited and crossover could have confounded these results.

Alexandra Thomas, MD, FACP
Importantly, PARP inhibitors were relatively well tolerated compared with single-agent chemotherapy, with quality-of-life analysis also favoring PARP inhibition, said Dr. Thomas. She noted that most adverse events were of a low grade, and dose reduction or supportive care is recommended.
Although talazoparib did have higher rates of grade 3 or 4 hematologic toxicities relative to single-agent chemotherapy, rates of PARP inhibitor treatment discontinuation were low. Moreover, cumulative exposure with extended cycles of talazoparib did not increase the incidence or severity of anemia.
Of note, both olaparib and talazoparib are associated with the risk of myelodysplastic syndromes and acute myeloid leukemia, Dr. Thomas reported.
Tyrosine Kinase Inhibitor: Tucatinib
Tucatinib, the most recently approved tyrosine kinase inhibitor, showed a 46% reduction in the risk of disease progression and a 34% reduction in the risk of death when added to trastuzumab and capecitabine in the HER2CLIMB trial.6 Notably, tucatinib appeared to have activity in the central nervous system, said Dr. Thomas, improving overall survival for patients with brain metastases in the tucatinib arm.
Although the rates of diarrhea were overall higher with tucatinib, grade 3 diarrhea was infrequent in both arms. Any grade 3 adverse event was also slightly more common with tucatinib, but agent discontinuation was infrequent, said Dr. Thomas. Of note, tucatinib can cause mild creatinine elevations without changes in glomerular filtration.
Immune Checkpoint Inhibitor: Atezolizumab
Checkpoint blockade has also recently entered the armamentarium to treat triple-negative breast cancer. The IMpassion130 study demonstrated improved progression-free survival as well as a significant survival benefit in the first-line setting for metastatic triple-negative breast cancers that overexpress PD-L1, favoring combination treatment with atezolizumab and nab-paclitaxel over single-agent nab-paclitaxel.7
In this trial, the withdrawal of any agent due to adverse events approached 16% in the atezolizumab arm and was just over 8% in the placebo arm. Importantly, said Dr. Thomas, immune-mediated toxicities from checkpoint blockade can occur early, during, and even after treatment with these agents.
Antibody-Drug Conjugates
Three antibody-drug conjugates are currently available to treat breast cancer, with many more in development. Although this type of precision targeting is appealing and is often therapeutically effective, said Dr. Thomas, toxicities remain a concern with this class of agents. The side-effect profile of antibody-drug conjugates—including ocular concerns that have been seen with several agents in development—can mimic that of their antineoplastic cargo, or they can be less common.8,9
“Having been approved several years ago for advanced disease, ado-trastuzumab emtansine (T-DM1) is familiar to us now,” said Dr. Thomas. “With this agent, rates of left-ventricular dysfunction are similar to those with single-agent trastuzumab, and similar monitoring is recommended.”
T-DM1 is also associated with frequent low-grade hepatotoxicity and thrombocytopenia, although rare deaths from severe manifestations have occurred. Notably, patients on T-DXd can experience hemorrhage in the absence of thrombocytopenia or anticoagulation, said Dr. Thomas.
In the past few months, two new antibody-drug conjugates have been approved: fam-trastuzumab deruxtecan-nxki (T-DXd) and sacituzumab govitecan-hziy. As Dr. Thomas reported, T-DXd demonstrated a “remarkable” response rate and median progression-free survival in heavily pretreated patients with HER2-positive breast cancer.
“Unlike [T-DM1], [T-DXd] is commonly associated with adverse events seen with cytotoxic chemotherapy, including cytopenias, nausea, vomiting, diarrhea, and alopecia, suggesting off-target exposure to the antineoplastic payload,” said Dr. Thomas. “Patients treated on this agent were also at risk for significant interstitial lung disease.”
Finally, sacituzumab govitecan, which was approved in April 2020 for patients with triple-negative breast cancer, targets Trop-2 and carries an irinotecan metabolite as cargo.10 Sacituzumab govitecan has an adverse-event profile that is similar to that of irinotecan and has black box warnings for severe diarrhea and severe neutropenia. According to Dr. Thomas, further understanding of the adverse-event profile will likely come in the postmarketing period.
“In this era of rapidly emerging drugs and drug classes, it will be critical to follow recommended monitoring and to collectively remain vigilant for postmarket effects, such as the pulmonary toxicity described years after the initial approval of CDK inhibitors,” Dr. Thomas concluded. “It will also be important to develop and maximize the use of predictive biomarkers and response-based imaging to further optimize agent dose and timing for our patients.”
DISCLOSURE: Dr. Thomas disclosed financial relationships with BeyondSpring Pharmaceuticals, Bristol Myers Squibb, Genentech, Gilead Sciences, Johnson & Johnson, Pfizer, Syndax, and Up-to-Date.
REFERENCES
1. Thomas A: What’s the price? Toxicities of targeted therapy in breast cancer care. ASCO20 Virtual Education Program.
2. Giuliano M, Schettini F, Rognoni C, et al: Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: A systematic review and network meta-analysis. Lancet Oncol 20:1360-1369, 2019.
3. André F, Ciruelos E, Rubovszky G, et al: Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 380:1929-1940, 2019.
4. Robson M, Im SA, Senkus E, et al: Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 377:523-533, 2017.
5. Litton JK, Rugo HS, Ettl J, et al: Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 379:753-763, 2018.
6. Murthy RK, Loi S, Okines A, et al: Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med 382:597-609, 2020.
7. Schmid P, Adams S, Rugo HS, et al: Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108-2121, 2018.
8. Verma S, Miles D, Gianni L, et al: Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783-1791, 2012.
9. Von Minckwitz G, Huang CS, Mano MS, et al: Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 380:617-628, 2019.
10. Bardia A, Mayer IA, Vahdat LT, et al: Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med 380:741-751, 2019.

