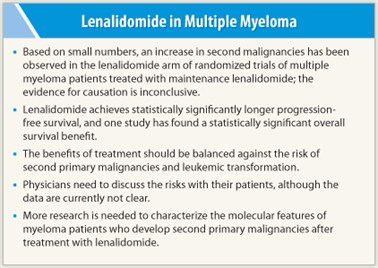
Three randomized controlled trials presented at the 2010 Annual Meeting of the American Society of Hematology (ASH) suggested that treating multiple myeloma with lenalidomide (Revlimid) increased the risk of second primary malignancies; of particular concern is transformation to acute myeloid leukemia or myelodysplastic syndromes.1-3 At the ASCO Annual Meeting in June, additional studies that explored this issue suggested that the risk of second primary malignancies in this setting is low and the benefits of lenalidomide seem to outweigh the risks, but a number of questions remain unresolved.
“Currently, we lack clear answers as to which patients will develop second primary malignancies after myeloma,” said Ola Landgren, MD, PhD, Chief of the Multiple Myeloma Section at NCI and formal discussant of the three papers at the ASCO meeting. “We lack clear answers due to small numbers of patients in these studies and limitations of the study designs. We have to discuss benefits vs risks with patients, but there are no clear data to guide us at this time. A concerted effort is needed to characterize the molecular features of patients who develop second primary malignancies after myeloma.”
MM-015 Study
 MM-015 was one of the three studies at ASH raising concern about second primary malignancies.3 At ASCO, an updated analysis related to second primary malignancies was presented. MM-015 enrolled 459 newly diagnosed, transplant-ineligible patients with a median age of about 71 years at 82 centers in Europe, Australia, and Israel.4 Patients with newly diagnosed multiple myeloma were randomly assigned to (1) melphalan/prednisone/lenalidomide followed by lenalidomide maintenance, or (2) melphalan/prednisone/lenalidomide, or (3) melphalan/prednisone and treated until disease progression, at which point they all received lenalidomide plus dexamethasone.
MM-015 was one of the three studies at ASH raising concern about second primary malignancies.3 At ASCO, an updated analysis related to second primary malignancies was presented. MM-015 enrolled 459 newly diagnosed, transplant-ineligible patients with a median age of about 71 years at 82 centers in Europe, Australia, and Israel.4 Patients with newly diagnosed multiple myeloma were randomly assigned to (1) melphalan/prednisone/lenalidomide followed by lenalidomide maintenance, or (2) melphalan/prednisone/lenalidomide, or (3) melphalan/prednisone and treated until disease progression, at which point they all received lenalidomide plus dexamethasone.
The continuous lenalidomide arm (arm 1) achieved a significant and “unprecedented” improvement in progression-free survival (median = 31 vs 13 months, respectively; 60% reduced risk of progression; P < .001). Progression-free survival was even more impressive in patients aged 65 to 75 years (69% reduced risk of progression; P < .001). However, no difference in 5-year survival was seen among the three arms. Presenting author of this paper, Antonio Palumbo, MD, University of Torino, Italy, said that with longer follow-up, a survival difference may emerge.
At a median follow-up of 30 months, the number of invasive second primary malignancies was 12 (7 hematologic malignancies, 5 solid tumors) in the lenalidomide maintenance arm of the trial, 9 (5 hematologic malignancies, 4 solid tumors) in the melphalan/prednisone/lenalidomide arm, and 4 (1 hematologic malignancy, 3 solid tumors) in the melphalan/prednisone arm.
Dr. Palumbo emphasized that the risk of second primary malignancies, although increased by the concomitant use of lenalidomide and melphalan, is low and that the benefit-risk profile strongly favors the use of continuous lenalidomide for newly diagnosed patients.
BiRD Study
 The BiRD study also explored this issue.5 BiRD was a small phase II trial involving 72 patients, median age 63 years, with newly diagnosed multiple myeloma. Patients were treated with lenalidomide, clarithromycin, and dexamethasone until progressive disease, stem cell transplantation, or unacceptable toxicity. At baseline, 8 patients had prior solid tumors, 2 had skin cancers, and 1 had lymphoma.
The BiRD study also explored this issue.5 BiRD was a small phase II trial involving 72 patients, median age 63 years, with newly diagnosed multiple myeloma. Patients were treated with lenalidomide, clarithromycin, and dexamethasone until progressive disease, stem cell transplantation, or unacceptable toxicity. At baseline, 8 patients had prior solid tumors, 2 had skin cancers, and 1 had lymphoma.
At a median follow-up of 6 years, median progression-free survival was 70.8 months and median 4-year overall survival was 82.2%; median 5-year overall survival had not yet been reached. Follow-up at 5 years revealed five new primary solid tumors and no new hematologic malignancies.
Progression-free survival remains encouraging; frequency of second primary malignancies was low and similar to that reported in the Surveillance, Epidemiology, and End Results (SEER) database for people of similar age, said presenting author Adriana Rossi, MD, Weill-Cornell Medical College, New York. “Routine screening and prevention measures should continue as medically indicated for each patient, including examination for skin cancer,” Dr. Rossi noted.
MM990/010 Study
MM990/010 was a pooled analysis of two phase III studies with a total of 704 patients with relapsed/refractory multiple myeloma treated with lenalidomide plus dexamethasone or placebo plus dexamethasone until disease progression.6
During the active treatment phase, 8 invasive second primary malignancies developed in the lenalidomide arm vs 2 in the placebo arm; 11 noninvasive and 2 noninvasive second primaries developed in the two arms, respectively. Moreover, 3 of the 8 invasive malignancies occurred within the first 13 months of treatment.
“No clear pattern of invasive second primaries was seen. Nonmelanoma skin cancers developed in 11 patients on lenalidomide plus dexamethasone and 2 patients on placebo,” said presenting author Ruben Niesvizky, MD, Weill-Cornell Medical College, New York.
The incidence of second primary malignancies was low and similar to the background incidence of similarly aged persons in the general population. However, overall survival was significantly longer in the group treated with lenalidomide plus dexamethasone, and improvement persisted with long-term follow-up. ■
Disclosure: Dr. Palumbo has served as a consultant for and received honoraria from Celgene and Janssen-Cilag, and has also received honoraria from Ortho Biotech. Dr. Rossi reported no potential conflicts of interest. Dr. Niesvizky has served as an advisor or consultant for Celgene, Millenium, and Onyx, has received honoraria from Celgene and Millenium, has received research funding from Proteolix, Seattle Genetics, Onyx, Millenium, and Celgene, has served on a data monitoring committee for Proteolix, and on an advisory committee/board of directors for Millenium and Celgene.
Expert Point of View: Second Primary Malignancies Explored in Multiple Myeloma
References
1. Attal M, Laywers VC, Marit G, et al: Maintenance treatment with lenalidomide after transplantation for MYELOMA: Final analysis of the IFM 2005-02. 52nd ASH Annual Meeting. Abstract 310. Presented December 6, 2010.
2. McCarthy PL, Owzar K, Anderson KC, et al: Phase III Intergroup study of lenalidomide versus placebo maintenance therapy following single autologous hematopoietic stem cell transplant for multiple myeloma: CALGB 100104. 52nd ASH Annual Meeting. Abstract 37. Presented December 5, 2010.
3. Palumbo AP, Delforge M, Catalano J, et al: A phase 3 study evaluating the efficacy and safety of lenalidomide combining melphalan and prednisone in patients ≥ 65 year with newly diagnosed multiple myeloma (NDMM): Continuous use of lenalidomide vs fixed-duration regimens. 52nd ASH Annual Meeting. Abstract 622. Presented December 6, 2010.
4. Palumbo AP, Delforge M, Catalano J, et al: The incidence of second primary malignancies (SPM) in melphalan-prednisone-lenalidomide combination followed by lenalidomide maintenance (MPR-R) in newly diagnosed multiple myeloma patients (pts) aged 65 or older. 2011 ASCO Annual Meeting. Abstract 8007. Presented June 5, 2011.
5. Rossi AC, Mark TM, Jayabalan D, et al: Incidence of second primary malignancies (SPM) after 6-years follow-up of continuous lenalidomide in first-line treatment of multiple myeloma (MM). 2011 ASCO Annual Meeting. Abstract 8008. Presented June 5, 2011.
6. Dimoupoulos MA, Orlowski RZ, Niesvizky R, et al: Lenalidomide and dexamethasone (LEN plus DEX) treatment in relapsed refractory myeloma (RRMM) patients and risk of second primary malignancy (SPM): Analysis of MM-009/010. 2011 ASCO Annual Meeting. Abstract 8009. Presented June 5, 2011.

 Formal discussant Ola Landgren, MD, PhD, Chief of the Multiple Myeloma Section at NCI, had some additional comments about the 2011 ASCO presentations on second primary malignancies in lenalidomide-treated patients.1 He said that the reporting of second primaries has several limitations that...
Formal discussant Ola Landgren, MD, PhD, Chief of the Multiple Myeloma Section at NCI, had some additional comments about the 2011 ASCO presentations on second primary malignancies in lenalidomide-treated patients.1 He said that the reporting of second primaries has several limitations that...