In the phase II GeparNuevo trial, patients with early triple-negative breast cancer receiving the PD-L1 inhibitor durvalumab in addition to chemotherapy as neoadjuvant therapy saw improvements in long-term outcomes. The results were presented at the 2021 ASCO Annual Meeting by Sibylle Loibl, MD, PhD, Chief Executive Officer and Chair of the German Breast Group and Associate Professor at the University of Frankfurt.1

Sibylle Loibl, MD, PhD
As previously reported by the group, pathologic complete response rates were 53.4% with durvalumab vs 44.2% in the placebo arm (adjusted odds ratio = 1.53; P = .182).2 In the latest analysis, after a median follow-up of 43.7 months, the 3-year invasive disease–free survival was 85.6% in the durvalumab arm vs 77.2% in the placebo arm (hazard ratio [HR] = 0.48; P = .0398). Distant disease–free survival and overall survival were also significantly improved.
“The [pathologic complete response rate] was increased by a small amount, 9%, which was not statistically significantly different,” although some subgroups did benefit more, Dr. Loibl said. The lack of significant benefit stands in contrast to findings from two other phase III trials in which the addition of a PD-1/PD-L1 inhibitor enhanced neoadjuvant therapy: the IMpassion031 trial, which evaluated atezolizumab plus chemotherapy,3 and the KEYNOTE-522 trial, which evaluated pembrolizumab plus chemotherapy.4
In those two trials, patients receiving the checkpoint inhibitors had pathologic complete response rates that were 17% and 14% higher, respectively, than patients receiving chemotherapy alone. Overall survival was also significantly improved (HR = 0.76 and 0.63, respectively), but median follow-up in those studies was relatively short (median = 21 and 16 months), Dr. Loibl pointed out.
About GeparNuevo
GeparNuevo randomly assigned 174 patients to receive durvalumab plus chemotherapy or chemotherapy alone. Patients in the durvalumab arm received 2 weeks of durvalumab, then durvalumab on day 1 of every 28-day cycle. After biopsy, they continued on durvalumab plus 125 mg/m2 of nab-paclitaxel weekly for 12 weeks. Patients achieving a clinical response then continued on durvalumab plus epirubicin and cyclophosphamide for 8 weeks, followed by surgery. The control arm received the same chemotherapy without durvalumab and no durvalumab for the 2-week lead-in period.
There were 34 invasive disease–free survival events: 12 in the durvalumab arm and 22 in the placebo arm. The 3-year invasive disease–free survival rate was 85.6% in the durvalumab arm vs 77.2% in the placebo arm (HR = 0.48; P = .0398). Distant disease–free survival was 91.7% vs 78.4% (HR = 0.31; P = .0078), and 3-year overall survival was 95.2% and 83.5%, respectively (HR = 0.24; P = .0108). An invasive disease–free survival subgroup analysis was also performed, revealing no notable differences, though the hazard ratio of 0.436 (P = .063) for the PD-L1–positive subgroup is “hypothesis generating,” Dr. Loibl commented.
Examination according to the achievement of a pathologic complete response found that patients responding completely to durvalumab had a 3-year invasive disease–free survival rate of 95.5%, whereas the nonresponders’ rate was 76.3%. These rates in the placebo cohort were 86.1% and 69.7%, respectively (HR = 0.34 for pathologic complete response vs not; P = .004). All durvalumab-treated patients achieving a pathologic complete response were alive without distant disease progression at 3 years.
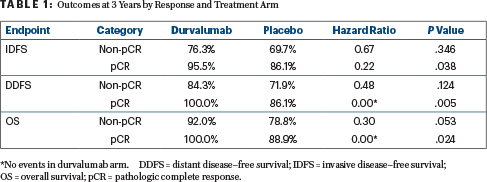
“Similar results were seen for the invasive disease–free survival, distant disease–free survival, and overall survival analyses,” Dr. Loibl noted (Table 1). “There were absolutely no distant disease–free survival events in the durvalumab arm as well as no deaths. This was overall statistically significantly different. The hazard ratio for all of those time-to-event endpoints between pathologic complete response and nonpathologic complete response was also statistically significantly different.”
DISCLOSURE: Dr. Loibl has received honoraria from Chugai Pharma; has served as an institutional consultant or advisor to AbbVie, Amgen, AstraZeneca/MedImmune, Bayer, Bristol Myers Squibb, Celgene, Daiichi Sankyo, EirGenix, GlaxoSmithKline, Immunomedics, Lilly, Merck KGaA, Novartis, Pfizer, Pierre Fabre, Prime/Medscape, Puma Biotechnology, Roche, Samsung, and Seattle Genetics; has received institutional research funding from AbbVie, Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Immunomedics Novartis, Pfizer, and Roche; and holds institutional intellectual property in “EP14153692.0.”
REFERENCES
1. Loibl S, Schneeweiss A, Huober JB, et al: Durvalumab improves long-term outcome in TNBC: Results from the phase II randomized GeparNuevo study investigating neoadjuvant durvalumab in addition to an anthracycline/taxane based neoadjuvant chemotherapy in early triple-negative breast cancer. 2021 ASCO Annual Meeting. Abstract 506. Presented June 7, 2021.
2. Loibl S, Untch M, Burchardi N, et al: A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 30:1279-1288, 2019.
3. Harbeck N, Zhang H, Barrios CH, et al: IMpassion031: Results from a phase 3 study of neoadjuvant atezolizumab plus chemotherapy in early triple-negative breast cancer. ESMO Virtual Congress 2020. Abstract LBA11. Presented September 20, 2020.
4. Schmid P, Cortes J, Pusztai L, et al: Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382:810-821, 2020.

