Although NTRK gene fusions occur in less than 5% of gastrointestinal cancers, it looks like they can be targeted successfully with NTRK inhibitors. In a pooled analysis of three clinical trials, 50% of such patients responded to entrectinib, in an updated analysis presented during the 2020 virtual edition of the European Society for Medical Oncology (ESMO) World Congress on Gastrointestinal Cancer.1 In another study presented during the meeting, a two-step diagnostic method identified NTRK fusions in up to 1% of patients with pancreaticobiliary tract cancers.2
“This updated analysis demonstrated that entrectinib induces clinically meaningful and durable systemic responses in patients with a range of NTRK fusion–positive gastrointestinal cancers,” said Manish R. Patel, MD, of the University of Minnesota, Minneapolis.

Manish R. Patel, MD
“NTRK1/2/3 gene fusions as well as ROS1 and ALK rearrangements are bona fide driver mutations for a variety of different cancers,” and they can be targeted, Dr. Patel commented. Entrectinib is an oral NTRK, ROS1, and ALK inhibitor and appears to be effective against a range of NTRK fusion–positive tumor types. The drug has shown clinically meaningful and durable responses in NTRK-positive tumors.
Updated Pooled Analysis
Dr. Patel presented an updated analysis of a large cohort of patients with gastrointestinal cancers harboring NTRK fusions. There were 74 patients who received more than one dose of entrectinib, pooled from three clinical trials: STARTRK-2, STARTRK-1, and ALKA-372-001. All patients had locally advanced or metastatic NTRK fusion–positive, NTRK inhibitor–naive tumors. Within this group were 16 patients with gastrointestinal cancers (mostly colorectal), all of whom were described in the safety analysis, and 12 patients were evaluated for efficacy, in the study reported during the meeting by
Dr. Patel.
Two-thirds of patients had NTRK1 fusions, and one-third had NTRK3 fusions. None of the patients had intracranial metastases at baseline; 50% had received two to three prior lines of therapy, indicating the study population was heavily pretreated.
‘Clinically Meaningful’ Benefit Seen
“Clinically meaningful responses were observed with entrectinib, with the vast majority of patients achieving significant tumor regression,” Dr. Patel reported. Of the 12 patients, 6 achieved a partial response, for a 50% objective response rate.
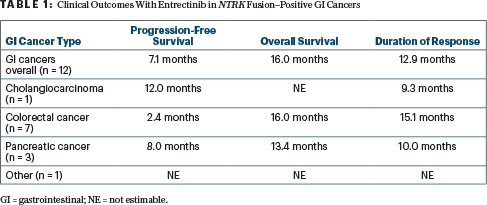
Responses were rapid and durable, and most patients demonstrated a response “early on,” noted Dr. Patel. The median duration of response was 12.9 months, with several patients demonstrating even greater durability of response. One patient remained on treatment at data cutoff. Table 1 shows all clinical outcomes (median values) from the three pooled clinical trials.
Tolerability
“Entrectinib was very well tolerated, with the most common side effect being taste change,” Dr. Patel reported. Grade 1 or 2 dysgeusia was observed in 37% of patients. All other toxicities were of a low grade, though some weight gain was observed, and it was grade 3 in two patients. One sudden death occurred, and its relationship with entrectinib could not be ruled out.
The vast majority of treatment-related adverse events resolved with dose reductions, which were required in three patients. Three other patients had doses interrupted, and one patient discontinued treatment.
“The results suggest we should be screening patients for NTRK fusions much more frequently,” Dr. Patel said. “Though they are rare, if we can identify patients with NTRK fusions during the course of disease, we can offer them benefits with entrectinib.”
Two-Step Testing Advocated
In a study presented by Anne Demols, MD, PhD, of CUB Hôpital ERASME in Brussels, the incidence of NTRK gene fusions in biliopancreatic cancers was indeed low: 0.67%.2

Anne Demols, MD, PhD
The study assessed the incidence of NTRK fusions in a retrospective population of biliary tract cancers and pancreatic adenocarcinomas. Investigators examined formalin-fixed paraffin-embedded archival tissue from intrahepatic, extrahepatic, perihilar, and gallbladder tumors and from pancreatic adenocarcinomas. They screened these samples by immunohistochemistry; if staining were positive, they subjected the sample to next-generation sequencing RNA-based analysis using the Oncomine Focus assay.
From the 162 biliary tract tumor samples, 149 were considered suitable for immunohistochemistry testing. Of them, 17 were immunohistochemistry-positive (most with weak staining), most commonly intrahepatic biliary tract tumors (n = 9). By next-generation sequencing, only a single sample (a poorly differentiated perihilar tumor) revealed an NTRK gene fusion, yielding an overall percentage of 0.67%, reported Dr. Demols.
Of the 319 archival samples of pancreatic adenocarcinomas, 297 were suitable for immunohistochemistry testing. Of them, 19 stained positive (most with weak staining), and next-generation sequencing testing of these samples revealed no NTRK gene fusions.
“Consistent with the low reported frequency of NTRK gene fusions in solid tumors, we found that NTRK gene fusions are also rare in biliopancreatic cancers,” Dr. Demols said. “Even with this low frequency, testing and identification are of clinical importance, due to their possible treatment with pan-NTRK inhibitors.”
“Next-generation sequencing is mandatory to confirm a positive immunohistochemistry test,” added Dr. Demols. She further suggested the two-step diagnostic approach, as applied in this study, “is time-saving and has an economic impact.”
DISCLOSURE: Dr. Patel has served on the advisory board of Nektar Therapeutics and has received research funding from Merck Sharpe and Dohme and Fate Therapeutics. Dr. Demols has served as a consultant or advisor to Bayer, Ipsen, Vitor, Servier, and Roche and has received research funding from Bayer.
REFERENCES
1. Patel MR, Siena S, Dimetri G, et al: Efficacy and safety of entrectinib in NTRK fusion-positive gastrointestinal cancers: Updated integrated analysis of three clinical trials (STARTRK-2, STARTRK-1 and ALKA-372-001). ESMO World Congress on Gastrointestinal Cancer 2020 Virtual. Abstract Oral-3.
2. Demols A, Perez-Casanova L, Rocq L, et al: NTRK gene fusions in biliopancreatic cancers. ESMO World Congress on Gastrointestinal Cancer 2020 Virtual. Abstract Oral-4.

