Patients with lower-risk myelodysplastic syndromes (MDS) who are dependent on red blood cell transfusions have limited options, especially if they are no longer responding to erythropoiesis-stimulating agents. Research presented during the virtual edition of the 25th European Hematology Association (EHA) Annual Congress suggested that two novel agents may prove to be beneficial in these patients.
Red blood cell transfusion independence was achieved by the telomerase inhibitor imetelstat in the phase II IMerge trial1 and by the oral hypomethylating agent CC-486 in the phase III QUAZAR study2 of patients with lower-risk MDS. Both studies met their primary endpoints.
Of note, the oral hypomethylating combination cedazuridine plus decitabine, formerly ASTX727, was approved in July 2020 by the U.S. Food and Drug Administration (FDA) for MDS and chronic myelomonocytic leukemia.
Phase II IMerge Trial of Imetelstat
Imetelstat, a first-in-class telomerase inhibitor, led to transfusion independence, often long-lasting, in patients with lower-risk MDS that is refractory to erythropoiesis-stimulating agents, in the phase II IMerge study, reported by Uwe Platzbecker, MD, of the University Clinic Leipzig, Germany.1 “Imetelstat achieved an 8-week transfusion independence rate of 42% for a median duration of 20 months, the longest so far reported with any agent in non-del(5q) low-risk MDS,” Dr. Platzbecker said.
In this population of patients who were red blood cell transfusion–dependent and relapsed or refractory to erythropoiesis-stimulating agents, 29% achieved transfusion independence for more than 1 year. For one patient, transfusion independence stretched beyond 2 and a half years, he said. Furthermore, hematologic improvement (erythroid) was achieved by 68% of patients, lasting a median of 21 months, reported Dr. Platzbecker.

“Imetelstat achieved an 8-week transfusion independence rate of 42%, for a median duration of 20 months, the longest so far reported with any agent in non-del(5q) low-risk MDS.”— Uwe Platzbecker, MD
Tweet this quote
Imetelstat targets cells with short telomeres and active telomerase, characteristics observed in MDS across all disease stages. It was evaluated in the global phase II/III IMerge study in patients with transfusion-dependent low-risk MDS who were relapsing on or refractory to erythropoiesis-stimulating agents. Patients received imetelstat at 7.5 mg/kg intravenously every 4 weeks.
IMerge: Patient Population
Dr. Platzbecker reported results from the open-label, single-arm phase II portion of IMerge, which included 38 patients with low-risk MDS who had non-del(5q) disease. All were naive to lenalidomide and hypomethylating agents. His presentation focused on long-term efficacy and safety and an analysis of biomarkers.
Long-term efficacy outcomes were red blood cell transfusion independence for at least 8 weeks, at least 24 weeks, and at least 1 year; the longest and cumulative duration of at least 8-week independence; duration of hematologic improvement (erythroid); and major/minor response per updated International Working Group (IWG) 2018 guidelines.
All patients had a high transfusion burden (≥ 8 U of red blood cells per 16 weeks and ≥ 4 U per 8 weeks). In addition, 84% had ≥ 6 U per 8 weeks; 89% had received prior erythropoiesis-stimulating agents; 32% had an erythropoietin level higher than 500 U/L; and 71% had ringed sideroblasts.
IMerge: Transfusion Independence Achieved by 42%
“The key findings were the high rates of transfusion independence and hematologic improvement (erythroid) in a patient population that was relapsed or refractory to erythropoiesis-stimulating agents and had a high transfusion burden. These responses were durable,” Dr. Platzbecker reported.
Across all MDS subgroups, the benefit of imetelstat treatment was observed. This included patients with mutations associated with a poor prognosis. At a median follow-up of 24 months, 16 of 38 patients (42%) achieved transfusion independence for at least 8 weeks, and 12 had a hemoglobin rise over baseline of at least 3 g/dL during the transfusion-free interval (Table 1).
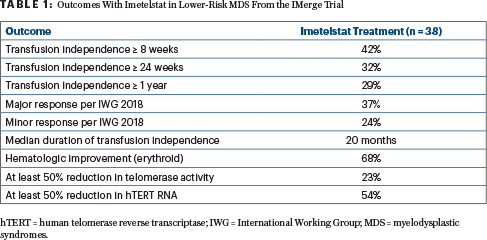
Furthermore, 12 patients (32%) achieved at least 24-week transfusion independence, and 11 patients (29%) whose median transfusion burden was 6 U per 8 weeks were transfusion-free for at least 1 year. The median duration of transfusion independence was 20 months, the longest being 2.7 years. Hematologic improvement (erythroid) was achieved by 26 patients (68%), with a median duration of 21 months.
The most frequently reported adverse events were grade ≥ 3 cytopenias, a known side effect of imetelstat. These events were manageable and reversible, and “the vast majority did not influence the study procedure,” Dr. Platzbecker said.
IMerge: Translational Research Shows Disease-Modifying Activity
Cytogenetic and mutational malignant clone reduction in some patients indicated the disease-modifying activity of imetelstat. Post-dosing on-target imetelstat activity was demonstrated by a reduction in telomerase activity of at least 50% in 23.1% of patients and of human telomerase reverse transcriptase RNA level in 54.3%. A correlation was shown between inhibition of the telomerase target with imetelstat and clinical benefit (ie, transfusion independence).
Enrollment is ongoing in the placebo-controlled phase III portion of IMerge, which will evaluate efficacy and safety of the drug as well as potential biomarkers of response.
Phase III QUAZAR Lower-Risk MDS Trial: Oral CC-486
In its primary analysis, the phase III QUAZAR Lower-Risk MDS trial of the novel oral hypomethylating agent CC-486 met its primary endpoint, showing a significant improvement in red blood cell transfusion independence vs placebo.2 The population included patients with intermediate risk-1 MDS but high-risk disease features, including severe red blood cell transfusion dependency and thrombocytopenia.
This is a population with a poor prognosis whose treatment options are limited, and this is the type of patients who benefited from CC-486 in this trial, said Guillermo Garcia-Manero, MD, of The University of Texas MD Anderson Cancer Center, Houston. “CC-486 met the primary endpoint of red blood cell transfusion independence and induced durable bilineage hemoglobin and platelet improvements,” he announced.

“CC-486 met the primary endpoint of red blood cell transfusion independence and induced durable bilineage hemoglobin and platelet improvements.”— Guillermo Garcia-Manero, MD
Tweet this quote
CC-486, which was formerly referred to as “oral azacitidine,” has a pharmacokinetic and pharmacodynamic profile that is distinct from injectable azacitidine. In extended dosing—for instance, 14 or 21 days of every 28-day cycle—CC-486 conferred therapeutic activity across the entire cycle, according to Dr. Garcia-Manero.
QUAZAR: Trial Details
All patients completing the study had intermediate-1 MDS according to the International Prognostic Scoring System, and red blood cell transfusion dependency plus thrombocytopenia, which was defined by ≥ 2 U per 28 days for 56 days and two platelet counts ≤ 75 × 109/L taken at least 21 days apart.
Patients were randomly assigned to CC-486 at 300 mg or placebo daily for 21 days per each 28-day cycle. The primary endpoint was red blood cell transfusion independence lasting at least 56 days (by IWG 2006 criteria).
An imbalance of early deaths in the CC-486 arm led to a decision to close enrollment. The final sample size of 216 patients allowed comparison of red blood cell transfusion independence between treatment arms with 99% power.
At data cutoff, all patients had completed at least 12 months of treatment or discontinued treatment before 12 months. Baseline characteristics were similar between treatment arms. Median transfusion requirement at baseline was 6.7 U over 56 days.
QUAZAR: Primary Endpoint Met
Significantly more patients in the CC-486 arm achieved red blood cell transfusion independence for at least 56 days: 31%, vs 11% (odds ratio [OR] = 3.6; P = .0002). The median duration of transfusion independence was 11.0 and 5.0 months, respectively (P = .42). Extending this to the 84-day analysis showed transfusion independence in 28% of the CC-486 arm but just 6% of placebo recipients (OR = 6.6; P < .0001). “The study, therefore, reached its primary endpoint,” Dr. Garcia-Manero reported.
Although hematologic improvement (erythroid) rates were comparable, significantly more CC-486 patients had a hemogloblin increase of at least 1.5 g/dL (P < .0001). Statistical significance was also seen for hematologic improvement (platelets) with CC-486 (24% vs 6%; OR = 4.6; P = .0003). The study sample size was underpowered for the interim overall survival analysis, which showed no difference between CC-486 and placebo (median 17.3 vs 16.7 months; P = .88).
“The death rate between the arms was similar, but it’s important to note there were more early deaths with CC-486: 16 vs 6 with placebo between days 1 and 56,” Dr. Garcia-Manero said. “Most of these deaths were related to infection, and the patients who died on the CC-486 arm had a median baseline absolute neutrophil count of 0.57/L and a platelet count of 15.5/L.”
“As expected, the patients treated with CC-486 had more adverse events, but they were managed with appropriate monitoring, especially during the early treatment cycles, with supportive care measures and dose modifications or interruptions as needed,” he indicated. Dr. Garcia-Manero cautioned that patients with severe neutropenia are at high risk for hematologic toxicity early during CC-486 treatment and may benefit from a modified dosing regimen and extensive best supportive care, including prophylactic antibiotics.
DISCLOSURE: Dr. Platzbecker has received honoraria from Celgene/Jazz; has served as a consultant or advisor to Celgene/Jazz; has received institutional research funding from Amgen, BerGenBio, Celgene, Janssen, and Novartis; holds a patent for a TRF-2 antibody; and has been reimbursed for travel, accommodations, or other expenses by Celgene. Dr. Garcia-Manero has received honoraria from AbbVie, Acceleron Pharma, Astex Pharmaceuticals, Celgene, and Helsinn; has served as a consultant or advisor to Acceleron Pharma, Astex Pharmaceuticals, Bristol Myers Squibb, Celgene, Helsinn Therapeutics, and Jazz Pharmaceuticals; and has received research funding from AbbVie, Amphivena Therapeutics, Astex Pharmaceuticals, Bristol Myers Squibb, Celgene, H3 Biomedicine, Helsinn Therapeutics, Merck, Novartis, and Onconova Therapeutics.
REFERENCES
1. Platzbecker U, Fenaux P, Steensma DP, et al: Treatment with imetelstat provides durable transfusion independence in heavily transfused non-del(5q) lower risk MDS relapsed/refractory to erythropoiesis stimulating agents. EHA25 Virtual Congress. Abstract S183.
2. Garcia-Manero G, Santini V, Almeida A, et al: A phase III placebo-controlled trial of CC-486 in patients with red blood cell transfusion-dependent anemia and thrombocytopenia due to IPSS lower-risk myelodysplastic syndromes. EHA25 Virtual Congress. Abstract S180.

