
Syed Ali Abutalib, MD

Sonali M. Smith, MD, FASCO
As part of The ASCO Post’s coverage of the 2019 ASCO Annual Meeting, featured here are four abstracts from different clinical trials evaluating newer treatments for Waldenström’s macroglobulinemia and T-cell lymphomas.
Waldenström’s Macroglobulinemia
ABSTRACT 7509: Outcomes with bendamustine plus rituximab (BR; n = 67); dexamethasone, rituximab, and cyclophosphamide (DRC; n = 75); and bortezomib, dexamethasone, and rituximab (BDR; n = 30) as front-line therapy in Waldenström’s macroglobulinemia: Single-institution retrospective study1
Background: Waldenstrom’s macroglobulinemia is a rare indolent lymphoma subtype that has shared clinical and biologic features of both non-Hodgkin lymphoma and plasma cell disorders. A number of regimens have been tested in mainly single-arm phase II trials, with no information on comparative activity. Given the rarity of the disease, prospective comparative trials of these commonly used regimens are unlikely to be performed, and thus this retrospective analysis (by investigators from the Mayo Clinic) is instructive.
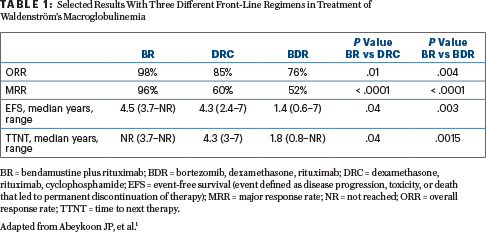
Results: Relevant endpoints and study results are shown in Table 1. Hematologic and nonhematologic toxicities were similar across the three treatment groups. Grade 3 neuropathy requiring treatment discontinuation was observed in 13% of patients treated with the BDR regimen.
Clinical Implications: The overall response rate, event-free survival, and time to next therapy all favored the BR regimen. In this retrospective analysis, front-line BR was superior to DRC and BDR. Clinically relevant endpoints were not significantly different between DRC and BDR. The toxicity profile across the three treatment groups was comparable.
T-Cell Lymphomas
ABSTRACT 7503: First-line therapy of T-cell lymphoma: Allogeneic or autologous hematopoietic cell transplantation (HCT) for consolidation—Final results of the AATT study2
Background and Methods: Peripheral T-cell lymphomas are rarely cured with initial therapy, and consolidative autologous HCT (auto-HCT) has evolved as a method of prolonging initial treatment response and attempting to improve outcomes. AATT was a prospective randomized trial comparingauto-HCT
In our opinion, given the high treatment-related mortality of allo-HCT, patients with T-cell lymphomas should not receive this modality as first-line consolidation therapy.— Syed Ali Abutalib, MD, and Sonali M. Smith, MD, FASCO
Tweet this quote
( n = 41) with allogeneic HCT (allo-HCT; n = 26) in younger (aged 18–60 years) transplant-eligible patients with newly diagnosed peripheral T-cell lymphoma (PTCL) who had achieved complete response, partial response, or stable disease after four courses of CHOEP (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone) and one course of DHAP (dexamethasone, cytarabine, and cisplatin). Patients were to receive BEAM (carmustine, etoposide, cytarabine, and melphalan) followed by auto-HCT or myeloablative conditioning (fludarabine, busulfan, and cyclophosphamide) followed by allo-HCT from an HLA-matched related or unrelated donor.
Primary Endpoint: 3-year event-free survival
Results: The median observation time for event-free survival was 42 months. The 3-year event-free survival and overall survival did not significantly differ between allo-HCT and auto-HCT (event-free survival: 43% [95% confidence interval (CI) = 29%–57%] vs 38% [CI = 25%–52%]; P = .58, overall survival: 57% [CI = 43%–71%] vs 70% [CI = 57%–82%; P = .41]). Comparing the 41 patients who actually received auto-HCT with the 26 patients who received allo-HCT, there was no significant difference in event-free survival, progression-free survival, and overall survival. No patient relapsed, but eight patients died of treatment-related mortality after allo-HCT compared with 13 relapses but no treatment-related mortality was observed after auto-HCT. Comparison of patients with an age-adjusted International Prognostic Index of 2 and 3 vs 0 and 1 showed significant differences for all endpoints.
Clinical Implications: The AATT study showed no survival difference between upfront allo-HCT and auto-HCT in patients with PTCL. However, it is worth noting that more patients failed to receive planned allo-HCT than auto-HCT (46% vs 24%, respectively). Allo-HCT resulted in substantially greater treatment-related mortality. In our opinion, given the high treatment-related mortality of allo-HCT, patients with T-cell lymphomas should not receive this modality as first-line consolidation therapy.
ABSTRACT 7538: Analysis of brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone by CD30 expression (defined as ≥ 10% by local review) in subset of patients treated on ECHELON-2 trial3
Background: The ECHELON-2 study demonstrated significantly longer progression-free and overall survival with brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone vs cyclophosphamide, doxorubicin, vincristine, and prednisone in the front-line treatment of patients with CD30-positive PTCL.4
Methods: The investigators in this multi-institutional trial analyzed the relationship between CD30 expression (above and below the median) and complete response rate, objective response rate, and duration of complete response in 37 patients with angioimmunoblastic T-cell lymphoma (AITL) and 34 patients with peripheral T-cell lymphoma–not otherwise significant (PTCL-NOS) treated on the experimental arm.
Results: In this particular analysis, CD30 levels were neither predictive of response nor significantly associated with the duration of complete response in patients with AITL (P = .30) or PTCL-NOS (P = .90; log-rank test; Table 2).
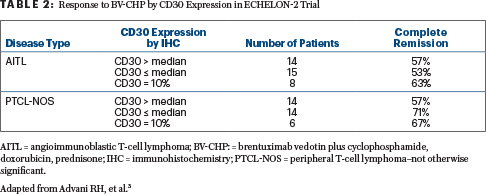
Clinical Implications: In patients with AITL and PTCL-NOS, the CD30 expression above vs below the median (or at minimum of 10%) did not predict response to brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone. However, the overall efficacy of this combination regimen in these two subsets is less than what is seen in those with anaplastic large cell lymphoma. Further evaluation of the expression-response relationship in patients with PTCL who have CD30 expression of less than 10% is warranted.
ABSTRACT e19031: Efficacy of mogamulizumab in previously treated patients with less advanced (stage IB/IIA) mycosis fungoides: Post hoc analysis of the MAVORIC study5
Background: In the phase III MAVORIC study, patients with previously treated mycosis fungoides/Sezary syndrome stage IB to IVB who received mogamulizumab had significantly prolonged progression-free survival and a better objective response rate than did patients treated with vorinostat.6
Methods: In the post hoc analysis of this multinational trial, the time to next treatment was defined as the time to any therapy excluding topical steroids or focal radiation treatment. The objective response rate was based on the global composite response in four disease compartments—skin, blood, lymph nodes, and viscera—achieved at two consecutive visits at least 8 weeks apart. Individual compartment responses were also assessed.
Results: A total of 85 patients with stage IB/IIA mycosis fungoides were included (mogamulizumab, IB n = 15, IIA n = 21; vorinostat, IB n = 27, IIA n = 22). Overall, 24% (10 of 42) of patients with stage IB disease and 28% (12 of 43) of patients with stage IIA disease had received at least six prior therapies.
Further evaluation of the expression-response relationship in patients with PTCL who have CD30 expression of less than 10% is warranted.— Syed Ali Abutalib, MD, and Sonali M. Smith, MD, FASCO
Tweet this quote
The median time to the next treatment with mogamulizumab in patients with stage IB disease was 11.5 months (95% CI = 1.4–16.0 months) compared with 3.1 months (95% CI = 2.7–5.3 months) with vorinostat; in patients with stage IIA disease, the median time to the next treatment was 10.1 months (95% CI = 5.5–12.6 months) and 4.9 months (95% CI = 2.4–8.0 months), respectively. The objective response rate in patients with stage IB disease receiving mogamulizumab and vorinostat was 20% (3 of 15) and 18.5% (5 of 27), respectively; the objective response rate in patients with stage IIA disease was 19% (4 of 21) and 0% (0 of 22), respectively. Adverse events were generally manageable and consistent with those in the intent-to-treat population.
Clinical Implications: Early mycosis fungoides (stage IB/IIA) is a chronic skin malignancy that can involve blood and nodes and may require many lines of systemic therapy over the disease course. Although the MAVORIC trial was not powered to determine treatment effect by disease stage, this post hoc analysis of the time to next treatment, objective response rate, and compartmental response in stage IB/IIA disease demonstrates a meaningful clinical benefit with mogamulizumab (compared with vorinostat) in previously treated patients with early mycosis fungoides. ■
Dr. Abutalib is Associate Director, Hematology and Cellular Therapy Program; Director, Clinical Apheresis Program; Cancer Treatment Centers of America, Zion, Illinois; Associate Professor, Roseland Franklin University of Medicine and Science; Founder and Co-Editor, Advances in Cell and Gene Therapy. Dr. Smith is Elwood V. Jensen Professor of Medicine; Interim Chief, Section of Hematology/Oncology; Director, Lymphoma Program; The University of Chicago, Chicago.
DISCLOSURE: Dr. Abutalib is an advisor for AstraZeneca. Dr. Smith has served in a consulting or advisory role for AbbVie/Genentech, AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Gilead Sciences, Kite Pharma, Pharmacyclics, Portola Pharmaceuticals, Seattle Genetics, and TG Therapeutics; and has received research funding from Acerta Pharma/AstraZeneca, Celgene, and Pharmacyclics/Janssen.
REFERENCES
1. Abeykoon JP, Zanwar S, Ansell SM, et al: Outcomes with rituximab plus bendamustine, dexamethasone, rituximab, cyclophosphamide, and bortezomib, dexamethasone, rituximab as primary therapy in patients with Waldenstrom macroglobulinemia. 2019 ASCO Annual Meeting. Abstract 7509. Presented June 3, 2019.
2. Shmitz N, Truemper L, Ziepert M, et al: First-line therapy of T-cell lymphoma: Allogeneic or autologous transplantation for consolidation—Final results of the AATT study. 2019 ASCO Annual Meeting. Abstract 7503. Presented June 4, 2019.
3. Advani RH, Horwitz SM, Iyer SP, et al: Response to A+CHP by CD30 expression in the ECHELON-2 trial. 2019 ASCO Annual Meeting. Abstract 7538. Presented June 3, 2019.
4. Horwitz S, O’Connor OA, Pro B et al: Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase III trial. Lancet 393:229-240, 2019.
5. Geskin LJ, Scarisbrick J, Bagot M, et al: Efficacy of mogamulizumab in previously treated patients with less advanced mycosis fungoides: Results from the MAVORIC study. 2019 ASCO Annual Meeting. Abstract e19031. Online only.
6. Kim YH, Bagot M, Pinter-Brown L, et al: Mogamulizumab vs vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase III trial. Lancet Oncol 19:1192-1204, 2018.

