The addition of a checkpoint inhibitor to standard chemotherapy as first-line treatment of advanced endometrial cancer reduced the risk of disease progression or death by 70% in patients with mismatch repair–deficient (dMMR) tumors in two recent phase III studies. The results of the two late-breaking trials, both reported at the Society of Gynecologic Oncology (SGO) 2023 Annual Meeting on Women’s Cancer, are poised to change the standard of care.
The ENGOT-EN6-NSGO/GOG-3031/RUBY trial1 and the NRG GY018/KEYNOTE-868 trial2 each tested a different inhibitor of PD-1—dostarlimab-gxly and pembrolizumab, respectively—given in combination with paclitaxel and carboplatin (and as maintenance treatment) in patients with previously untreated advanced or recurrent endometrial cancer. Efficacy was evaluated in all patients and in the subpopulation with dMMR tumors.

Rebecca Arend, MD, MPH
“Both trials hit a home run,” according to the studies’ invited discussant, Rebecca Arend, MD, MPH, Associate Professor at the University of Alabama and Associate Scientist in the Experimental Therapeutics Program at O’Neal Comprehensive Cancer Center, Birmingham. “Those of us who do research, whether it be in the lab or in the clinic, have all dreamed of being part of something that actually changes clinical care. Congratulations to everyone who contributed to making this happen,” Dr. Arend said.
Investigators had hypothesized that the addition of cytotoxic chemotherapy could improve the response to checkpoint inhibitors, since it has multiple immunogenic effects: inhibition of myeloid-derived suppressor cells, increased antigen cross-presentation following immunogenic cell death, enhanced dendritic cell activity via STAT6 pathway inhibition, and an increased ratio of cytotoxic lymphocytes to regulatory T cells.
The results of both the GY018/KEYNOTE-868 and RUBY trials were published simultaneously in The New England Journal of Medicine.3,4 The RUBY trial was also presented at the European Society for Medical Oncology (ESMO) Virtual Plenary on March 27, 2023.5
Dostarlimab in GOG-3031/RUBY Trial
“This is the biggest news for patients with endometrial cancer in more than 30 years,” said Mansoor Mirza, MD, Chief Oncologist at Rigshospitalet, Copenhagen University Hospital in Denmark. “Dostarlimab plus carboplatin and paclitaxel represents a new standard of care for patients with primary advanced or recurrent endometrial cancer.”

Mansoor Mirza, MD
Dr. Mirza continued: “These results have shown an unprecedented improvement in progression-free survival as a result of adding immunotherapy to standard -chemotherapy, especially in the 25% of patients with so-called hot endometrial tumors that have deficient DNA mismatch-repair -mechanisms.” He also noted a “clinically meaningful long-term benefit” in patients with mismatch repair–proficient (pMMR) or microsatellite-stable (MSS) tumors and an “early overall survival trend” in the entire study population.
RUBY enrolled 494 patients with primary stage III (18.6%), stage IV (33.6%), or recurrent (47.8%) endometrial cancer. About one-quarter of patients (23.9%) had dMMR/microsatellite instability–high (MSI-H) disease, and nearly half had recurrent disease.
Patients were randomly assigned in a 1:1 ratio to receive dostarlimab at 500 mg or placebo in combination with carboplatin at an area under the curve (AUC) of 5 mg/mL/min and paclitaxel at 175 mg/m2 every 3 weeks for the first six cycles, followed by dostarlimab at 1,000 mg or placebo every 6 weeks for up to 3 years. The trial had a multiplicity control strategy, for which the primary endpoints were progression-free survival in the dMMR/MSI-H population and the intention-to-treat population and overall survival in the intention-to-treat population (for which the statistical boundary was P = .00177) by investigator assessment.
Broad Benefit With Dostarlimab
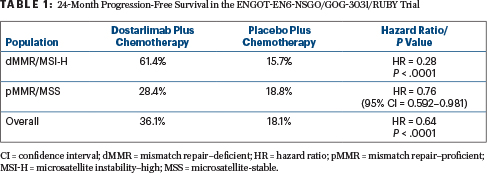
At a median follow-up of 25 months, dostarlimab plus chemotherapy reduced the risk of disease progression or death by 72% in patients with dMMR/MSI-H disease (hazard ratio [HR] = 0.28; P < .0001) and by 36% in the entire study population (HR = 0.64; P < .0001). Median progression-free survival was not reached with dostarlimab and chemotherapy and was 7.7 months with chemotherapy alone in the dMMR/MSI-H population; it was 11.8 months vs 7.9 months, respectively, in the overall population (Table 1).
Dr. Mirza emphasized that in the dMMR/MSI-H population, the addition of immunotherapy yielded a 72% benefit, “which was much larger than the 50% we expected to see.” In the pMMR/MSS subset, the effect, he said, was “moderate.”
At 33% maturity, overall survival data showed a trend favoring dostarlimab; median overall survival was not reached in either arm, but at 24 months, 83.3% of the dostarlimab dMMR/MSI-H group remained alive vs 58.7% of the placebo group (HR = 0.30; 95% confidence interval [CI] = 0.127–0.699); for the overall population, these rates were 71.3% vs 56.0%, respectively (HR = 0.64; P = .0021), and for the pMMR/MMS group, they were 67.7% vs 55.1% (95% CI = 0.515–1.024).
“This difference was seen despite the fact that 34.5% of patients in the placebo arm received immune therapy after disease progression, as did 15.5% of patients in the dostarlimab arm,” Dr. Mirza said.
Objective response rates were not much different: 77.6% for dostarlimab/chemotherapy and 69.0% for chemotherapy alone in the dMMR/MSI-H population and 68.1% vs 63.4% for pMMR/MSS patients. However, the duration of response was notably prolonged with dostarlimab, where the median was not reached for the dMMR/MSI-H subset.
No new safety signals were seen with the drugs used in the trial. Approximately 17% of patients discontinued treatment in the dostarlimab arm, as did 9% in the placebo arm. Patient-reported quality of life was notably improved among patients treated with dostarlimab/chemotherapy, for both the dMMR/MSI-H and overall populations.
Pembrolizumab in GY018/KEYNOTE-868
Ramez N. Eskander, MD, of the University of California San Diego Moores Cancer Center, presented interim efficacy data from the international phase III NRG GY018/KEYNOTE-868 trial of 816 patients with stage III/IVA, IVB, or recurrent endometrial cancer. Within this population were 225 patients with dMMR tumors and 591 with pMMR tumors who were randomly assigned to paclitaxel at 175 mg/m2 plus carboplatin at AUC 5 every 3 weeks plus either pembrolizumab at 200 mg or placebo for six cycles; this was followed by pembrolizumab at 400 mg or placebo every 6 weeks for up to 14 additional cycles. The primary endpoint was progression-free survival by investigator review in dMMR and pMMR populations. Importantly, the dMMR and pMMR populations were evaluated independently as part of NRG GY018.

Ramez N. Eskander, MD
With a median follow-up of 12 months, the addition of pembrolizumab to chemotherapy resulted in a 70% reduction in the risk of disease progression or death in the dMMR population. Patients receiving pembrolizumab plus chemotherapy had a median progression-free survival that was not reached, compared with 7.6 months for the control arm (HR = 0.30; P < .00001). Progression-free survival rate at 12 months was 74% vs 38%, respectively. A significant benefit was also observed in the pMMR population, where the median progression-free survival was 13.1 months with pembrolizumab/chemotherapy vs 8.7 months with chemotherapy alone (HR = 0.54; P < .00001).
“The efficacy curves separated early in the course of treatment, with a preserved separation throughout the evaluation period in both populations,” Dr. Eskander noted.
“Subgroup analysis showed a consistent benefit for pembrolizumab in combination with chemotherapy across all relevant subgroups, including patients who had received prior radiation; patients with prior systemic adjuvant chemotherapy; those with newly diagnosed, advanced stage, or recurrent disease; and those with more aggressive histologies,” he said. This was true for both the dMMR and pMMR analyses.
The addition of pembrolizumab to chemotherapy did not appear to increase the occurrence of adverse events commonly associated with carboplatin/paclitaxel chemotherapy, and there was not an excess in immune-mediated toxicities as compared with previous experience with pembrolizumab monotherapy.
Dr. Eskander commented: “We are hopeful that the above findings will transform standard of care treatment in the dMMR and pMMR patient populations, both of which had independent benefit with the addition of pembrolizumab to chemotherapy in the NRG GY018 clinical trial.”
DISCLOSURE: Dr. Arend has served as a consultant or advisor to Merck, Seagen, Sutro, GSK, VBL Therapeutics, and Kiyatec. Dr. Mirza reported financial relationships with AstraZeneca, Biocad, GSK, Merck, Roche, Zai Lab, Karyopharm, Ultimovacs, Apexigen, and Deciphera. Dr. Eskander has served as a consultant or advisor to AstraZeneca, Cardiff Oncology, Clovis Oncology, Eisai, Elevar Therapeutics, GSK/Tesaro, ImmunoGen, Seagen, Mersana, Myriad, Daiichi Sankyo, and Gilead Sciences.
REFERENCES
1. Mirza MR et al: Dostarlimab in combination with chemotherapy for the treatment of primary advanced or recurrent endometrial cancer. Society of Gynecologic Oncology 2023 Annual Meeting on Women’s Cancer. Presented March 27, 2023.
2. Eskander RM et al: Pembrolizumab versus placebo in addition to carboplatin and paclitaxel for measurable stage III or IVA, stage IVB, or recurrent endometrial cancer. Society of Gynecologic Oncology 2023 Annual Meeting on Women’s Cancer. Presented March 27, 2023.
3. Mirza MR et al: Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. March 27, 2023 (early release online).
4. Eskander RN et al: Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. March 27, 2023 (early release online).
5. Mirza MR et al: Dostarlimab + chemotherapy for the treatment of primary advanced or recurrent endometrial cancer. ESMO Virtual Plenary. Abstract VP2-2023. Presented March 27, 2023.

