In patients with myeloid or lymphoid neoplasms with FGFR1 rearrangements, pemigatinib produced high and durable response rates, despite patients’ extensive use of prior treatments or hematopoietic stem cell transplantation (HSCT), according to the early results of the multicenter phase II FIGHT-203 trial.1 At the 2021 American Society of Hematology (ASH) Annual Meeting & Exposition, the study was considered a “Best of ASH” abstract.
The selective inhibitor of fibroblast growth factor receptor (FGFR) receptors 1, 2, and 3 yielded a complete response rate of 64.5% and a complete cytogenic response rate of 72.7% by investigator assessment. Rates were even higher by central review—77.4% and 75.8%, respectively, according to Jason Gotlib, MD, MS, Professor of Medicine at Stanford Cancer Institute and Stanford University School of Medicine, who presented the findings.

Jason Gotlib, MD, MS
“The investigators conclude that pemigatinib is an effective treatment option for patients who are ineligible for or awaiting stem cell transplant,” Dr. Gotlib said.
Background
Myeloid or lymphoid neoplasms are rare hematologic cancers involving tyrosine kinase fusion genes and typically associated with eosinophilia. FGFR1-rearranged myeloid or lymphoid neoplasms may present with chronic or blast phase involvement of the bone marrow and/or involvement of extramedullary sites.
Standard therapy has not been established, but hydroxyurea, multikinase inhibitors with anti-FGFR1 activity (eg, midostaurin, ponatinib), or intensive multiagent chemotherapy may produce partial responses or short-lived complete responses. However, complete cytogenetic responses are rare, and the prognosis is poor.
“Given the poor prognosis of FGFR1-rearranged myeloid or lymphoid neoplasms and the potential for transformation to blast phase, a primary treatment goal is to achieve deep responses to bridge patients to HSCT,” Dr. Gotlib said.
Pemigatinib is approved in the United States, European Union, and Japan for the treatment of adults with previously treated, unresectable, locally advanced, or metastatic cholangiocarcinoma with an FGFR2 fusion or other rearrangement. The current study, the ongoing phase II FIGHT-203 trial, is evaluating pemigatinib in adults with FGFR1-rearranged myeloid or lymphoid neoplasms.
Study Details
FIGHT-203 initially required patients to have received at least one prior therapy; the starting dosing of pemigatinib was 13.5 mg daily for 2 weeks on, 1 week off. The protocol was amended to allow patients without prior therapy, and the starting dose was changed to 13.5 mg daily on a continuous schedule. The primary endpoint was investigator-assessed complete response rate.
Complete and partial cytogenetic responses were defined, respectively, as 100% or at least 50% reduction in 8p11-rearranged metaphases or cells on karyotyping or fluorescence in situ hybridization (FISH). Responses were also adjudicated retrospectively by a central review committee.
The efficacy analysis was based on 33 evaluable patients, 28 of whom (85%) had prior treatment of FGFR1-rearranged myeloid or lymphoid neoplasms. The average number of prior therapies was 1.6 (range = 0–6); three patients had prior HSCT.
Of these 33 evaluable patients, 7 patients had no morphologic evidence of disease; 23 patients had myeloid neoplasms, including 20 in chronic phase and 3 in blast phase; 2 patients had lymphoid neoplasms in blast phase; and 1 patient had mixed phenotype neoplasm in blast phase.
At baseline, 21% had no morphologic evidence of bone marrow involvement (but demonstrated a persistent FGFR1 rearrangement by standard cytogenetics and/or FISH); 70% had a myeloid neoplasm, 6% had a lymphoid neoplasm, and 3% had a mixed-phenotype acute leukemia. The most common FGFR1 fusion partner genes were 13q12/ZMYM2 (45.5%) and 22q11/BCR (24.2%).
The longest duration of pemigatinib exposure was 192.4 weeks, with a median dosing duration of 29.3 weeks. Patients completed a median of 10 cycles. At data cutoff, treatment was ongoing in 18 patients (53%), and for others, it was discontinued primarily for HSCT and progressive disease.
Response and Safety
Investigator-assessed complete responses and complete cytogenetic response rates were reported to be high (Table 1). Responses were less frequent and durable in patients in blast vs chronic phase.
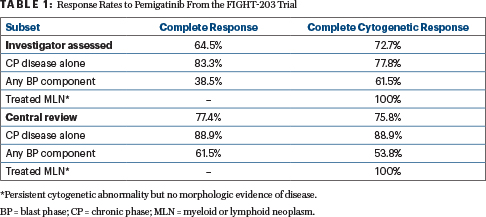
Among 18 patients with chronic phase disease alone, ongoing responses were reported by 12 patients at the time of data cutoff (range = 25–131 weeks). Among 11 patients with blast phase disease and response assessments, ongoing responses were reported in 2 patients at data cutoff (range = 8–177 weeks). Median duration of response for all patients has not been reached.
Safety was comparable to previous data for FGFR inhibitors. Grade ≥ 3 adverse events were observed in 85% of patients, including primarily anemia (18%), stomatitis (12%), pain in the extremities (12%), and thrombocytopenia (9%). The most common toxicities of any grade were hyperphosphatemia (68%), alopecia (59%), diarrhea (50%), and stomatitis (44%). Treatment-related adverse events led to discontinuation of therapy in four patients (for cardiac failure, multiple organ dysfunction, increased blood alkaline phosphatase, and calciphylaxis).
DISCLOSURE: Dr. Gotlib has received honoraria from and served as a consultant or advisory to Blueprint Medicines, Novartis, Deciphera, Incyte, Kartos, PharmaEssentia, Cogent Biosciences, and Allakos.
REFERENCE
1. Gotlib J, Kiladjian JJ, Vannucchi A, et al: A phase 2 study of pemigatinib (FIGHT-203; INCB054828) in patients with myeloid/lymphoid neoplasms with fibroblast growth factor receptor 1 rearrangement. 2021 ASH Annual Meeting & Exposition. Abstract 385. Presented December 12, 2021.

