
Ignace B. Vergote, MD, PhD
In the treatment of advanced endometrial cancer, maintenance therapy with oral selinexor after response to first-line chemotherapy may result in a significantly reduced risk of disease progression, according to the results of the global phase III ENGOT-EN5/GOG-3055/SIENDO trial, presented at the European Society for Medical Oncology (ESMO) Virtual Plenary March 2022 session by Ignace B. Vergote, MD, PhD, a gynecologic oncologist at the Catholic University of Leuven and the Cancer Institute at University Hospitals Leuven, Belgium.1
In the stratification factor–audited intent-to-treat population, maintenance selinexor resulted in a 30% decrease in the risk for disease progression and/or death compared with placebo (P = .024). More strikingly, patients with endometrioid histology had a 43% decrease in risk (P = .014), and those with TP53 wild-type tumors had a 62% risk reduction (P = .0003), Dr. Vergote reported.
Patients with metastatic or recurrent endometrial cancer are usually treated with paclitaxel and carboplatin, but most have short-term disease control and a poor prognosis. And although endometrial cancer is usually diagnosed at an early and curable stage, about 25% of these women will relapse within 5 years, and their prognosis is poor, said Isabelle Ray-Coquard, MD, PhD, Professor of Medical Oncology at the University Claude Bernard Lyon I and a medical oncologist at the Centre Léon Bérard, France, who provided context to the abstract in her introduction. “There has been no improvement in overall survival for 20 years,” she noted, adding that one way to improve outcomes may be to find an effective maintenance therapy.

Isabelle Ray-Coquard, MD, PhD
Selinexor is an oral selective inhibitor of exportin 1 (XPO1), the major nuclear export protein for tumor-suppressor proteins such as p53. Inhibition of XPO1 results in an increase in nuclear levels, activation of tumor suppressor proteins, and a reduction of oncoprotein levels. The drug has shown clinical activity in previously treated advanced endometrial cancer.
About the Trial
ENGOT-EN5/GOG-3055/SIENDO is a prospective, multicenter, double-blind, placebo-controlled phase III study that evaluated selinexor at 80 mg once weekly vs placebo as maintenance therapy in 263 patients with advanced or recurrent endometrial cancer who achieved partial or complete remission after treatment with a taxane plus carboplatin (the current standard of care). After achieving a complete or partial response to first-line chemotherapy, patients were randomly assigned 2:1 to selinexor at 80 mg weekly (n = 174) or placebo (n = 89) until disease progression. The primary endpoint was investigator-assessed progression-free survival.
The arms, which were well balanced, were stratified according to primary stage IV vs recurrent endometrial cancer and partial vs complete response. Median age was approximately 65, histology was endometrioid in about 54% and serous in about 30% of patients, slightly more than half the patients had recurrent disease, and about 43% achieved complete responses.
Marked Risk Reduction With Selinexor
After a median follow-up of 10.2 months, the stratification-adjusted analysis of progression-free survival showed a 30% reduction in risk with selinexor maintenance. Median progression-free survival was 5.7 months with selinexor and 3.8 months with placebo (hazard ratio [HR] = 0.705, 95% confidence interval [CI] = 0.499–0.996; P = .024).
“It’s important to note that the curves began to split at around 4 months, and then the difference between the arms persisted,” Dr. Vergote said. The percentage of patients progression-free was 48.2% vs 40.9% at 6 months; 41.7% vs 34.1% at 9 months; and 35.3% vs 25.8% at 12 months.
The progression-free survival in the intent-to-treat population was based on audited stratification factors. Dr. Vergote explained that in seven patients (2.7%), the stratification factor of complete vs partial response was incorrect at the time of randomization, but later it was corrected by the investigators prior to unblinding and analysis. The statistical analysis was independently validated and approved by the data safety and monitoring committee. Without correcting for this error, the investigators said the hazard ratio would be 0.76 (95% CI = 0.543–1.076) and not significant.
Most Benefit in Subsets
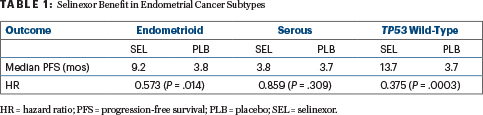
The prespecified exploratory analyses of subgroups revealed that the most robust benefit from maintenance selinexor was in patients with endometrioid histology and those with TP53 wild-type tumors (Table 1).
Dr. Vergote commented that the robust response in patients with TP53 wild-type tumors aligns with biology. “This is the group where we can expect the effect of selinexor,” he said, noting that selinexor reactivates p53 by preventing nuclear export. Additionally, about 80% of patients with TP53 wild-type tumors have endometrioid histology, and these tumors are also more likely to respond to the drug.
Adverse events were clearly more common with selinexor, but most were mild. Grade 3 toxicities associated with selinexor included nausea (10%), neutropenia (9%), thrombocytopenia (6%), fatigue (6%), asthenia (6%), anemia (5%), and abdominal pain (2%). One patient experienced a grade 4 thrombocytopenia, but there were no severe cases of bleeding. Approximately 10% of patients in the selinexor arm discontinued treatment due to toxicity, compared with 1% on the placebo arm. Patient-reported quality-of-life surveys (EORTC QLQ-C30) revealed no differences between the arms in global health, physical function, or symptoms, he added.
DISCLOSURE: Dr. Vergote disclosed financial relationships with Agenus, Akesobio, AstraZeneca, Bristol Myers Squibb, Deciphera Pharmaceuticals, Eisai, Elevar Therapeutics, F. Hoffmann–La Roche, Genmab, GSK, ImmunoGen, Jazz Pharmaceuticals, Karyopharm, Mersana, MSD, Novocure, Novartis, Oncoinvent AS, Seagen, Sotio, Tesaro, Verastem Oncology, and Zentalis. Dr. Ray-Coquard reported relationships with Agenus, Aksebio, AstraZeneca, Bristol Myers Squibb, Deciphera Pharmaceuticals, Eisai,F. Hoffmann–La Roche, Genmab, GSK, ImmunoGen, Mersana, MSD, Novocure, Novartis, Seagen, PharmaMar, MacroGenics, Adaptimmune, and Clovis.
REFERENCE
1. Vergote IB, Perez Fidalgo A, Hamilton E, et al: Prospective double-blind, randomized phase III ENGOT-EN5/GOG-3055/SIENDO study of oral selinexor/placebo as maintenance therapy after first-line chemotherapy for advanced or recurrent endometrial cancer. ESMO Virtual Plenary March 2022. Abstract VP2-2022. Presented March 17, 2022.


