A novel immunotherapeutic combination that targets PD-1 and the LAG-3 pathway significantly delayed disease progression as a first-line treatment of advanced or unresectable melanoma. Updated results of the global phase III RELATIVITY-047 trial validated the study’s initial findings and were presented in the ASCO Plenary Series, March 2022 session,1 by Georgina V. Long, MBBS, PhD, of the Melanoma Institute Australia, The University of Sydney, where she is Co-Medical Director and Chair of Melanoma Medical Oncology and Translational Research.

“These data further validate this combination [nivolumab plus relatlimab] as a potential new treatment option in patients with advanced melanoma and support the benefit of dual checkpoint inhibition.”— GEORGINA V. LONG, MD, PhD
Tweet this quote
In the study, nivolumab and relatlimab were given as a fixed-dose combination to 714 previously untreated patients with advanced or metastatic melanoma. The initial results were recently published2 and summarized in the April 10 issue of The ASCO Post. Dr. Long reported the updated progression-free survival data and the first data on the secondary endpoints of overall survival and response rate.
“At this analysis, with additional longer follow-up, nivolumab plus relatlimab continued to demonstrate a consistent -benefit in improving progression-free survival compared with nivolumab alone. The hazard ratio [HR] was 0.78, representing a 22% reduction in the risk of disease progression or death with the combination. The progression-free survival favored the nivolumab/relatlimab combination for all stratification factors, and overall survival rates were numerically improved at 12, 14, and 36 months vs nivolumab alone,” Dr. Long said. “These data further validate this combination as a potential new treatment option in patients with advanced melanoma and support the benefit of dual checkpoint inhibition.”
Rationale for the Combination
RELATIVITY-047 is the first phase III study of a novel fixed-dose combination to show a clinically meaningful benefit by dual inhibition of the LAG-3 and PD-1 pathways. LAG-3 upregulates an immune checkpoint pathway that inhibits T-cell activity. Relatlimab, a human IgG4 LAG-3–blocking antibody, restores the effector function of exhausted T cells and reinvigorates the T cells to attack the tumor. The combination of relatlimab and the anti–PD-1 agent nivolumab modulates potentially synergistic immune checkpoint pathways, and this is thought to enhance antitumor immune responses.
About RELATIVITY-047
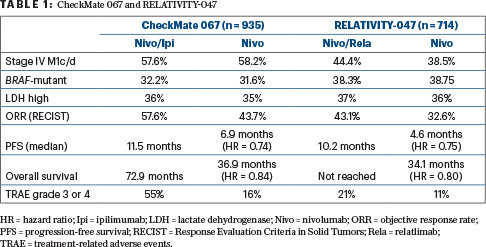
In the double-blind randomized trial, 714 previously untreated patients received a fixed dose of nivolumab at 480 mg plus relatlimab at 160 mg every 4 weeks or nivolumab at 480 mg alone. Approximately 40% of the population had M1c or M1d disease, 67% had a performance status of 0, and less than 10% had prior neoadjuvant or adjuvant therapy. At baseline, 36% had an elevated lactate dehydrogenase level, 75% had LAG-3 expression of at least 1%, 59% had PD-L1 expression of less than 1%, and 38% had BRAF mutations.
In the initial analysis, after a median follow-up of 13.2 months, the study met its primary endpoint. By blinded independent review, the combination significantly improved median progression-free survival from 4.6 months with nivolumab alone to 10.2 months (hazard ratio [HR] = 0.75; P = .0055).2
Initial Findings Upheld
In the updated analysis, after a median follow-up of 19.3 months, the outcomes were virtually identical to those of the initial analysis. Median progression-free survival was 10.2 months with relatlimab/nivolumab vs 4.6 months with nivolumab alone (HR = 0.78; 95% confidence interval = 0.64–0.94). The percentage of patients free of disease progression was 48.0% vs 36.9%, respectively, at 12 months and 38.5% vs 29.0% at 24 months, she reported.
Median overall survival was not reached with the combination vs 34.1 months with nivolumab alone (HR = 0.80; P = .0593). Overall survival rates were 77.0% vs 71.6%, respectively, at 12 months, 63.7% vs 58.3% at 24 months and 55.8% vs 48.8% at 36 months.
The overall survival boundary for statistical significance was P < .04302 (two-sided) based on 69% power for a target hazard ratio of 0.75. Therefore, statistical significance was not met, but the difference is “clinically meaningful,” with benefit observed across all stratification factors, she said.
“It is important to interpret these overall survival rate results in the context of subsequent therapy. Subsequent therapy occurred less often with the combination (40.8%) than with nivolumab monotherapy (42.6%). For example, effective systemic therapy was used less often with nivolumab/relatlimab,” Dr. Long added.
For the combination therapy vs the single agent, checkpoint inhibitors were included in 11.8% and 15.9%, respectively, and BRAF and/or MEK inhibitors, in 12.4% and 14.8%.
The combination also increased responses to 43.1% from 32.6% with nivolumab alone (complete response rates were 16.3% and 14.2%). The difference in the overall response rate was 10.3%, with an odds ratio of 1.6; the median duration of response was not reached in either arm, she said.
Safety Profile
Treatment-related adverse events were slightly increased with nivolumab/relatlimab (83.7% vs 72.4%), mostly pruritus, fatigue, and rash, but grade 3 or 4 toxicities were limited (21.1% vs 11.1%). There were four treatment-related deaths in the combination arm and two in the nivolumab arm.
Immune-mediated adverse events were also slightly more frequent with the combination (18.6% vs 14.8%), mostly thyroid abnormalities, rash, and diarrhea/colitis. Of note, myocarditis occurred in six patients (1.7%) given nivolumab/relatlimab and two patients (0.6%) given nivolumab alone. Troponin monitoring was done for the first 2 months of treatment, per protocol.
DISCLOSURE: Dr. Long has served as a consultant or advisor to or received honoraria from Agenus, Amgen, Array Biopharma, Boehringer Ingelheim, Bristol Myers Squibb, Evaxion, HexalAG (Sandoz Company), Highlight Therapeutics S.L., Merck Sharpe & Dohme (Australia) Pty Limited, Novartis Pharma AG, OncoSec, Pierre Fabre, Provectus, Qbiotics, and Regeneron.
REFERENCES
1. Long GV, Hodi S, Lipson EJ, et al: Relatlimab and nivolumab versus nivolumab in previously untreated metastatic or unresectable melanoma: Overall survival and response rates from RELATIVITY-047 (CA224-047). 2022 ASCO Plenary Series. Abstract 360385. Presented March 15, 2022.
2. Tawbi HA, Schadendorf D, Lipson EJ, et al: Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med 386:24-34, 2022.

