
Syed Ali Abutalib, MD

Matt Lunning, DO, FACP
Chimeric antigen receptor (CAR) T-cell therapies are a significant advance, but they require careful patient selection, dependency on patients’ own T cells, lymphodepleting chemotherapy, possible bridging therapy, manufacturing timelines with extensive health-care coordination and cost, in addition to the potential for serious adverse events (mainly high-grade [≥ 3] cytokine-release syndrome and immune effector cell–associated neurotoxicity syndrome [ICANS] along with prolonged cytopenias and infections). These clinical and logistic challenges have rekindled enthusiasm in the potentially less complex approach of bispecific antibodies. In theory, these agents offer off-the-shelf, immediate-availability products, with early studies noting comparable response rates to CAR T-cell therapy (sometimes even after CAR T-cell therapy failures), early signal of durable remissions (limited median follow-up), and palatable initial toxicity data.
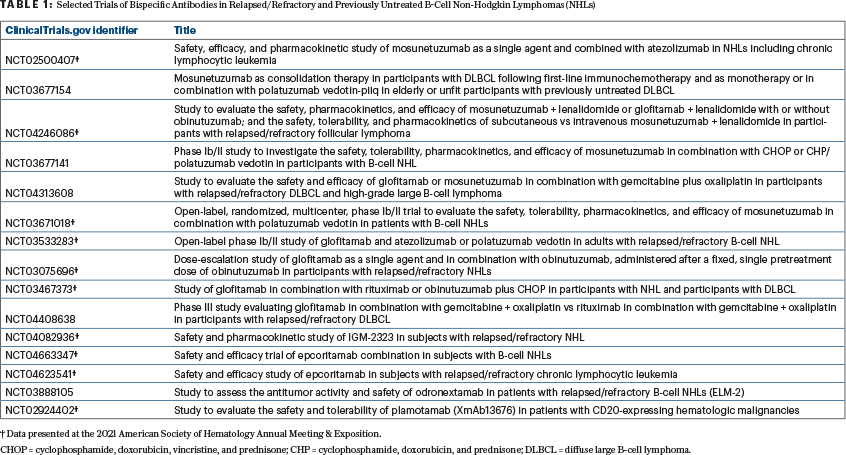
Consequently, numerous studies with bispecific antibodies in relapsed and refractory and previously untreated B-cell non-Hodgkin lymphoma (NHL) are ongoing. Several of these studies are listed in Table 1. Here, we highlight a recent Journal of Clinical Oncology report on a first-in-human study of single-agent mosunetuzumab1 and two abstracts on another bispecific antibody, glofitamab,2-4 from the 2021 American Society of Hematology Annual Meeting & Exposition.
Mosunetuzumab
Budde et al: Single-agent mosunetuzumab (n = 230) shows durable complete responses in participants with relapsed or refractory aggressive and indolent B-cell NHLs: Phase I dose-escalation study1 (ClinicalTrials.gov identifier NCT02500407).
Background: Mosunetuzumab targets CD3 and CD20, redirecting T cells to engage and eliminate malignant B cells. Preclinical studies have showed that mosunetuzumab induces rapid and sustained T-cell activation and proliferation as well as potent lysis of CD20-expressing B cells. Mosunetuzumab is an off-the-shelf product, requiring no time for manufacturing, whereas the manufacture of CAR T cells can be a process that takes up to 2 to 4 weeks after apheresis.
Methods:
Prior Lines of Therapy: In the diffuse large B-cell lymphoma (DLBCL) and transformed follicular lymphoma (FL) cohorts, subjects must have failed to respond to at least two prior systemic therapies (including anthracycline and anti-CD20–directed therapy). In the FL cohorts, subjects must have FL grade 1-3a and failed to respond to at least two prior systemic therapies (including anti-CD20–directed therapy and an alkylating agent).
Duration of Therapy: Mosunetuzumab was discontinued after 8 cycles for subjects with a complete response and after 17 cycles for those achieving a partial response or stable disease. Retreatment was permitted for subjects who relapsed after a complete response.
Dosing: The first-in-human international trial enrolled subjects into two groups receiving intravenous (IV), time-limited mosunetuzumab in 3-week cycles: 33 subjects received fixed doses of up to 2.8 mg on day 1 (group A); 197 subjects received ascending doses during cycle 1 on days 1, 8, and 15, with treatment continuing for 8 or 17 cycles based on tumor response (group B). In group B, dose escalation at 1/2/13.5 mg was evaluated in 43 subjects with FL. Thereafter, dosing was modified to 1/2/60 mg in cycle 1, 60 mg on day 1 of cycle 2, and 30 mg on day 1 of subsequent cycles (1/2/60/60/30 mg dosing scheme).
Results:
Safety: In group B (n = 197), common adverse events (≥ 20% of patients) were neutropenia (28.4%), cytokine-release syndrome (27.4%), hypophosphatemia (23.4%), fatigue (22.8%), and diarrhea (21.8%). Cytokine-release syndrome was mostly low grade (grade ≤ 3: 1.0%; Lee criteria) and mainly confined to cycle 1, with 1.5% of subjects requiring tocilizumab, an interleukin 6 antagonist, and 1.0% of patients requiring intensive care unit admission. The spectrum and severity of neurologic adverse events observed in this study, including mostly grade 1 or 2 headache, insomnia, and dizziness, also compare favorably with the toxicities of encephalopathy and delirium reported with CD19-directed CAR T-cell therapies. The adverse-event profile for patients who had received prior CAR T-cell therapy was similar to that in the overall population.
“Mosunetuzumab achieved durable complete responses in subjects with aggressive and indolent relapsed or refractory B-cell non-Hodgkin lymphomas.”— Syed Ali Abutalib, MD, and Matt Lunning, DO, FACP
Tweet this quote
Efficacy: Across the doses investigated (group B), best overall response rates were 34.9% and 66.2% in subjects with aggressive and indolent B-cell NHLs, respectively, and complete response rates were 19.4% and 48.5%. Among subjects with a complete response, the median duration of response was 22.8 months (95% confidence interval [CI] = 7.6 months to not estimable) and 20.4 months (95% CI = 16 months to not estimable) in subjects with aggressive and indolent B-cell NHLs, respectively.
Clinical Implications: When administered by step-up dosing, time-limited, IV, off-the-shelf CD3/CD20 bispecific immunotherapy with mosunetuzumab had a manageable safety profile, with a low frequency of high-grade cytokine-release syndrome rates mainly confined to cycle 1. Mosunetuzumab achieved durable complete responses in subjects with aggressive and indolent relapsed or refractory B-cell NHLs. Maturation of follow-up, including dose expansion in group B, is needed to better understand the treatment benefit. The expansion stage of the study is ongoing at the dose level of 1/2/60/60/30 mg.
Glofitamab
ABSTRACT 130: Time-limited step-up dosing of glofitamab induces high response rates in subjects with relapsed or refractory mantle cell lymphoma (MCL), most of whom (n = 17) had failed to respond to prior Bruton’s tyrosine kinase (BTK) inhibitor therapy.2
Background: Glofitamab is a T-cell–engaging, CD3 x CD20 bispecific antibody with a 2:1 molecular configuration with bivalency for CD20 on B cells and monovalency for CD3 on T cells. Glofitamab with obinutuzumab pretreatment has shown efficacy with frequent, durable complete responses and manageable tolerability with both fixed-dosing and step-up dosing in NHLs.3
Methods: IV step-up dosing of glofitamab (n = 18) was given on days 1 and 8 of cycle 1, then at the target dose from cycle 2 day 1 (from cycle 3 day 1 for step-up dosing starting at 0.5 mg), every 3 weeks for up to 12 cycles (0.5/2.5/10/30, 2.5/10/16, or 2.5/10/30 mg after 1,000 mg of obinutuzumab pretreatment or 2.5/10/30 mg after 2,000 mg of obinutuzumab pretreatment). Subjects on fixed dosing (n = 3) received glofitamab (0.6, 16, or 25 mg) after 1,000 mg of obinutuzumab pretreatment from cycle 1 for up to 12 cycles. Many subjects were refractory to their first line of therapy (52%; n = 15) and/or their last prior therapy (69%; n = 20). The median time since last therapy was 1.7 months (range, 0.1–107.5 months).
Results:
Efficacy (n = 21): the objective response rate was 81.0% (n = 17), and the metabolic complete response rate was 66.7% (n = 14). Similar response rates were observed in subjects who had received prior BTK inhibitor therapy vs subjects who had not. The median duration of complete response follow-up was 2.4 months (range, 0.0–25 months); 85.7% (12 of 14) for subjects with a complete response remained in remission at data cutoff (median duration of response and median duration of complete response were not reached).
Safety (n = 29): Most common adverse events were cytokine-release syndrome (58.6%) and infusion-related reactions (24.1%), likely related to dosing of obinutuzumab. All cytokine-release syndrome events were grade 1 or 2, except for one grade 4 cytokine-release syndrome in the 1,000-mg obinutuzumab pretreatment plus step-up dosing cohort. Cytokine-release syndrome rates were lower in the 2,000-mg obinutuzumab pretreatment plus step-up dosing cohort. Neurologic adverse events occurred in six subjects (20.7%, all grade 1 [n = 5] or grade 2 [n = 1]). ICANS-like adverse events occurred in one subject (3.4%). No subject discontinued treatment due to an adverse event.
Clinical Implications: Step-up dosing of glofitamab as monotherapy after obinutuzumab pretreatment to mitigate cytokine-release syndrome induced high response rates in subjects with MCL, most of whom had failed to respond to prior BTK inhibitor therapy. The rates of cytokine-release syndrome were manageable and mostly low grade. Neurotoxicity was infrequent and low grade and appears to have resolved within 1 day. These properties are in contrast with those of current approved CAR T-cell therapies, which require manufacturing, may require bridging therapy, and may not be feasible in patients with rapidly progressive disease.
ABSTRACT 128: Glofitamab as monotherapy (n = 53) and in combination with obinutuzumab (n = 19) induces high complete response rates in subjects with multiple relapsed or refractory FL.4
Background: Glofitamab is a T-cell–engaging, CD3 x CD20 bispecific antibody with a novel 2:1 molecular configuration with bivalency for CD20 on B cells and monovalency for CD3 on T cells.
Methods: Obinutuzumab (1,000 mg) was given 7 days prior to the first dose of glofitamab to mitigate the risk of cytokine-release syndrome. For the three monotherapy cohorts, IV, step-up dosing of glofitamab was given on days 1 and 8 of cycle 1: then at target dose on cycle 2, or as step-up dosing on cycle 1 days 1 and 8, cycle 2 day 1, and the target dose on cycle 3 day 1. For the combination cohort, step-up dosing of glofitamab was given on days 1 and 8 of cycle 1, then at a target dose combined with obinutuzumab at 1,000 mg from cycle 2 day 1 and onward (every 21 days for up to 12 cycles). Median age was 64 years (range, 33–83 years) in the monotherapy cohorts and 61 years (range, 41–78 years) in the combination cohort; the median number of prior therapies was three (range, 1–12) and two (range, 1–5), respectively.
Results:
Adverse Events: Cytokine-release syndrome was the most common adverse event; 66% and 79% in the monotherapy and combination cohorts, respectively. Neurologic adverse events were seen in 26 subjects (16 given monotherapy, 10 given combination therapy; 36%), and all grade 1 or 2. No ICANS-like events related to glofitamab were reported. Other common adverse events were infusion-related reactions and cytopenias.
Monotherapy Cohort (n = 53): The objective response rate was 81%, and the metabolic complete response rate was 70%. Cytokine-release syndrome events were mostly grade 1 (47.2%) and 2 (17.0%); one subject had a grade 3 event. Tocilizumab was used to treat cytokine-release syndrome in 22.9% of subjects.
Combination Cohort (n = 19): The objective response rate was 100%, and the metabolic complete response rate was 73.7%. There were no grade 3 cytokine-release syndrome events in the combination cohort. Tocilizumab was used to treat cytokine-release syndrome in 33.3% of patients.
Clinical Implications: Step-up dosing with glofitamab administered as monotherapy or in combination with obinutuzumab achieved high response rates in subjects with heavily pretreated relapsed and refractory FL, including high-risk subgroups. Response rates were comparable to those reported for CAR T-cell therapy in relapsed and refractory FL. The safety profile of glofitamab was manageable; cytokine-release syndrome events were mostly low grade and occurred mainly in the first two cycles. Longer follow-up is required to further assess the durability of glofitamab in this heterogeneously treated population.
DISCLOSURE: Dr. Abutalib has served on the advisory board for AstraZeneca. Dr. Lunning reported relationships with AbbVie, Acrotech, ADC Therapeutics, Astellas, AstraZeneca, BMS, Daiichi Sankyo, EUSA, Fate Therapeutics, Genentech, Genmab, InstilBio, Kite, Morphosys, Novartis, Nurix Therapeutics, Sana Therapeutics, and TG Therapeutics.
REFERENCES
1. Budde LE, Assouline S, Sehn LH, et al: Single-agent mosunetuzumab shows durable complete responses in patients with relapsed or refractory B-cell lymphomas: Phase I dose-escalation study. J Clin Oncol 40:481-491, 2022.
2. Phillips T, Dickinson M, Morschhauser F, et al: Glofitamab step-up dosing induces high response rates in patients with relapsed or refractory mantle cell lymphoma, most of whom had failed prior Bruton’s tyrosine kinase inhibitor therapy. 2021 ASH Annual Meeting & Exposition. Abstract 130. Presented December 11, 2021.
3. Hutchings M, Morschhauser F, Iacoboni G, et al: Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable completerRemissions in relapsed or refractory B-cell lymphoma: A phase I trial. J Clin Oncol 39:1959-1970, 2021.
4. Morschhauser F, Carlo-Stella C, Dickinson M, et al: Glofitamab as monotherapy and in combination with obinutuzumab induces high complete response rates in patients with multiple relapsed or refractory follicular lymphoma. 2021 ASH Annual Meeting & Exposition. Abstract 128. Presented December 11, 2021.
Dr. Abutalib is Director, Hematology and BMT/Cellular Therapy Programs; Director, Clinical Apheresis Programs; Cancer Treatment Centers of America, Zion, Illinois; Associate Professor, Rosalind Franklin University of Medicine and Science; and Founder and Co-Editor of Advances in Cell and Gene Therapy. Dr. Lunning is Associate Professor in the Division of Hematology/Oncology, Department of Internal Medicine, and Assistant Vice Chair of Research, Department of Medicine, University of Nebraska Medical Center, Omaha.

