The novel targeted agent CPI-0610 enhanced responses to ruxolitinib in patients with myelofibrosis enrolled in the global phase II MANIFEST-2 trial, investigators reported at the 2020 American Society of Hematology (ASH) Annual Meeting & Exposition.1,2
“Preliminary data demonstrate the potential for the combination of CPI-0610 and ruxolitinib to provide enhanced efficacy, as evidenced by greater reductions in spleen volume and total symptom score at week 24 compared with historical data from pivotal phase III studies,” said John Mascarenhas, MD, Associate Professor of Medicine at Icahn School of Medicine at Mount Sinai, New York.
When added to ruxolitinib therapy, CPI-0610 also enhanced responses in JAK inhibitor–exposed patients, reported Srdan Verstovsek, MD, PhD, Chief of the Section for Myeloproliferative Neoplasms at The University of Texas MD Anderson Cancer Center, Houston.

John Mascarenhas, MD

Srdan Verstovsek, MD, PhD
With ruxolitinib alone, he noted, “the majority of patients with myelofibrosis show suboptimal responses, and there is limited evidence of disease modification. In addition, grade 3 and higher anemia and thrombocytopenia are concerns associated with ruxolitinib. The outcomes for patients who discontinue the drug are poor, with a median survival of approximately 1 year. CPI-0610 may act synergistically in combination with ruxolitinib,” Dr. Verstovsek said.
CPI-0610 is a first-in-class, oral, small-molecule inhibitor of the bromodomain and extraterminal domain (BET) protein. The BET family of proteins binds to chromatin to regulate the transcription of genes related to multiple profibrotic pathways. In particular, CPI-0610 targets genes involved in dysregulated cytokine production that drives inflammation, extramedullary hematopoiesis, and bone marrow fibrosis, ultimately helping promote erythrocyte differentiation and normalization of megakaryocyte differentiation, according to Dr. Mascarenhas.
Phase II MANIFEST-2 Details
MANIFEST-2 is evaluating CPI-0610 both in combination with ruxolitinib in JAK inhibitor–naive patients and as an “add-on” therapy to ruxolitinib in patients with suboptimal response. Dr. Mascarenhas presented results for arm 3, which to date has included 78 patients without prior JAK inhibitor exposure now receiving the doublet.
Of these patients, 76% were classified by the Dynamic International Prognostic Scoring System (DIPSS) as intermediate-2 or higher; for comparison with other series, 33% of patients were classified by the International Prognostic Scoring System (IPSS) as intermediate-2 or higher and 50% as IPSS-high.
Patients’ median spleen volume was 1,719 cc, median hemoglobin was 9.1 g/dL, median platelet count was 294 × 109/L, and median total symptom score (TSS) was 16. The primary endpoint was spleen volume response ≥ 35% from baseline (SVR35) after 24 weeks. TSS ≥ 50% reduction (TSS50) was a key secondary endpoint.
Spleen Volume Reduction in 67%
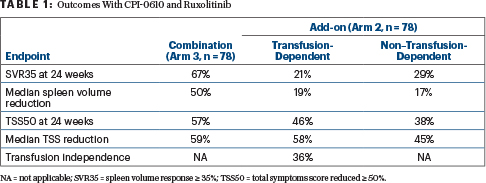
At 24 weeks, 67% of patients achieved SVR35, with a median volume reduction of 50%. Of patients with IPSS-2 or higher, SVR35 response was 66%. SVR35 was maintained for the duration of the 75-week follow-up, with progressive decreases in spleen volume observed out to week 60. This reflects a durability in response that is “perhaps more important than the frequency of achieving SVR35,” Dr. Mascarenhas said.
Patients remained relatively stable on their initial doses, with little need for up-titration. The mean daily dose at week 24 was 26 mg for ruxolitinib and 125 mg for CPI-0610. The most common dose of ruxolitinib was 10 mg twice daily, which was associated with SVR35 in 69%, a response that is comparable to those seen with higher ruxolitinib doses, he said.
Symptoms also improved for most patients, with TSS50 responses achieved by 57%, with a 59% median reduction in TSS at week 24. Mean hemoglobin dipped slightly at first but returned to baseline and above after three cycles. In patients with baseline hemoglobin < 10 g/dL and no transfusions, hemoglobin levels increased by about 1 g/dL over time.
Showing the impact of CPI-0610 on the disease process, the combination led to at least a one-grade improvement in bone marrow fibrosis in 33% of patients, with the vast majority improving within 6 months. Improvements in erythroid differentiation and megakaryocyte histotopography were also observed. “The improvement in bone marrow findings suggest potential disease modification with this approach,” Dr. Mascarenhas commented.
‘Add-on’ Therapy
Dr. Verstovsek reported the results of arm 2 of MANIFEST, which investigated CPI-0610 as add-on therapy in 78 patients with suboptimal or lost responses to ruxolitinib.2 Patients were stratified as transfusion-dependent (defined as requiring ≥ 2 units of red blood cells per month over 12 weeks; n = 52) and non–transfusion-dependent (n = 26). Ruxolitinib dose escalation was not allowed during the study.
The primary endpoint was conversion to transfusion independence in the transfusion-dependent cohort (no transfusion for 12 weeks) and SVR35 response at week 24 in the non–transfusion-dependent cohort. Patients’ median hemoglobin was 8.9 g/dL, median platelet count was 179 × 109/L, median spleen volume was 2,046 cc, and median TSS was 20. During the study, median ruxolitinib dose was 10 mg twice daily, median CPI-0610 dose was 125 mg daily, and median treatment duration was 45 weeks.
“Combination therapy provided clinical benefits in most transfusion-dependent patients as assessed by conversion to transfusion independence, spleen volume response, and symptomatic responses (Table 1). Spleen volume response and symptomatic benefit were also observed in non–transfusion-dependent patients,” Dr. Verstovsek said.
Transfusion independence was achieved by 36% of the transfusion-dependent cohort; its median duration was 27 weeks. Furthermore, overall transfusion intensity (number of units per month) also dropped after CPI-0610 was added to ruxolitinib.
The majority of patients experienced a decrease in spleen size and symptom improvement (Table 1). Bone marrow fibrosis also improved, by at least one grade in 41% of patients; most (81%) improved within 6 months of starting treatment.
Safety Profile
CPI-0610 in combination with ruxolitinib was generally well tolerated. Treatment-related adverse events grade ≥ 3 were recorded for 44% of arm 3 and 58% of arm 2. The most common hematologic toxicities were, respectively, anemia (33% and 14%; grade 3 or 4 in 29% and 11%) and -thrombocytopenia (32% and 45%: grade 3 or 4 in 8% and 26%). Thrombocytopenia was reported to be manageable and reversible.
The most common nonhematologic treatment-emergent adverse event was diarrhea, with some respiratory tract infections, nausea, asthenic conditions, cough, and taste changes. Discontinuation of treatment due to side effects was noted for 11 patients in both arms; 8 deaths occurred on treatment.
“Safety appears very good, with no major problems. The treatment does require some dose modifications, for sure, but overall, the benefits outpace any worry about added toxicity,” Dr. Verstovsek said.
Future Directions
Next, the phase III double-blind MANIFEST-2 trial will randomly assign 310 JAK inhibitor–naive patients to ruxolitinib plus CPI-0610 or placebo. The study will help answer the question of which patients most need the doublet by correlating baseline features with response and will determine whether the doublet produces more durable responses.
KEY POINTS
- The global phase II MANIFEST-2 trial is evaluating CPI-0610, a BET inhibitor, both in combination with ruxolitinib in JAK inhibitor–naive patients and as an “add-on” therapy to ruxolitinib in patients with suboptimal response to ruxolitinib.
- In ruxolitinib-naive patients, 67% of patients achieved a spleen volume reduction ≥ 35% (SVR35). As add-on therapy, SVR35 was achieved by 21% of transfusion-dependent and 29% of transfusion-independent patients.
- A ≥ 50% reduction in total symptom score was achieved by 57%, 46%, and 38%, respectively.
- There were several indications of a modification of the disease process.
“We know from multiple studies that the median time to discontinuing ruxolitinib is about 3 years and, after that, median overall survival is rather poor,” Dr. Mascarenhas said. “What we should be striving for is not simply to get more patients into SVR35 response but to extend that benefit. MANIFEST-2 will provide insights on this and will help determine which patients should get this combination.”
Dr. Verstovsek added: “Any agent, such as CPI-0610, that can enhance the ability to provide good management with JAK inhibitors themselves has the potential to be broadly used in the future.”
DISCLOSURE: Dr. Mascarenhas has received institutional research funding from Incyte, Roche, Forbius, CTI Bio, Merck, AbbVie, Novartis, Geron, Kartos, and Pharmaessentia; and has served in a consulting or advisory role for Celgene/BMS, Constellation Pharmaceuticals, Incyte, Novartis, Roche, Sierra, CTI Bio, Genentech/Roche, Gilead Sciences, Promedior, PharmaEssentia, Kartos, and AbbVie. Dr. Verstovsek has served as a consultant or advisor to Celgene, Constellation Pharmaceuticals, Incyte, Novartis, Pragmatist, and Sierra; and has received research funding from Blueprint Medicines, Celgene, CTI BioPharma Corp, Genentech, Gilead Sciences, Incyte, Novartis, NS Pharma, Promedior, and Roche.
REFERENCES
1. Mascarenhas J, Harrison C, Patriarca A, et al: CPI-0610, a bromodomain and extraterminal domain protein (BET) inhibitor, in combination with ruxolitinib, in JAK-inhibitor-naive myelofibrosis patients. 2020 ASH Annual Meeting & Exposition. Abstract 55. Presented December 5, 2020.
2. Verstovsek S, Mascarenhas J, Kremyanskaya M, et al: CPI-0610, bromodomain and extraterminal domain protein (BET) inhibitor, as “add-on” to ruxolitinib, in advanced myelofibrosis patients with suboptimal response: Update of the MANIFEST phase 2 study. 2020 ASH Annual Meeting & Exposition. Abstract 56. Presented December 5, 2020.


