Breast cancer screening and imaging-based surveillance after treatment remain suboptimal, largely because of confusion in the guidelines and the fact that dense breasts are too often ignored, according to Elizabeth Morris, MD, FACR, FSBI, FISMRM, Professor and Chair of the Department of Radiology at the University of California Davis. At the 2023 Miami Breast Cancer Conference, Dr. Morris offered her own recommendations for patients, including wider use of magnetic resonance imaging (MRI), and she predicted that the inevitable use of artificial intelligence (AI) in radiology will bring a new level of accuracy to these practices.1

Elizabeth Morris, MD, FACR, FSBI, FISMRM
Confusion on Current Guidelines
“Current guidelines for breast cancer screening are confusing, not only to patients but also to clinicians. Screening algorithms have been all over the place…. They are arbitrary and age-based,” Dr. Morris stated.
For population-based screening, for example, the American Cancer Society recommends annual mammography for women between the ages of 45 and 54 and mammography every other year starting at age 55. The U.S. Preventive Services Task Force recommends mammography for women aged 50 and older every other year. (The Task Force also recommends that women in their 40s talk to their clinician and make an individual decision about starting screening.) And the American College of Radiology recommends mammography every year, starting at age 40.
Patients are also not clear on their personal risk. Most assume they are at high risk for breast cancer only if they have a family history of the BRCA gene. However, more than 90% of breast cancers have no known genetic mutation, and more than 85% occur in patients with no known family history, Dr. Morris pointed out.
“I want to make people aware that until we get to personalized screening—and I hope that one day we do (trials are underway)—we are stuck with population-based screening, for the most part,” Dr. Morris continued. “Our goal is to pick up cancer at the very, very earliest possible stage, before it can develop genetic heterogeneity and have clonal changes. And to pick up the smallest cancers, we need to move to more contrast-based screening techniques. To this end, MRI detects more breast cancers up to 1 cm than any other screening test [using contrast, which reveals angiogenesis]. It rarely misses invasive cancers, it preferentially identifies more aggressive cancers, it lessens the risk of detecting cancer at advanced stages (especially in BRCA carriers), and it reflects the tumor’s molecular and genetic makeup.”
Dr. Morris emphasized: “With MRI, we are picking up the more biologically relevant cancers, whereas mammography tends to preferentially pick up the less biologically aggressive cancers. This is why we, in radiology, have always suspected that MRI screening should save lives.”
Proof of MRI’s Benefits
The Netherlands’ DENSE trial evaluated additional MRI screening in average-risk women with extremely dense breasts.2 It found the interval cancer rate to be 5.06 per 1,000 mammograms, dropping to 0.83 per 1,000 when the exam combined mammography with MRI. This was a pivotal trial showing that MRI screening does save lives, and its results led to a governmental recommendation that MRI screening should be offered to all women with extremely dense breasts, regardless of risk.
“The problem is that MRI is expensive, and we don’t have the capability, particularly in the United States, to use it for screening every woman with very dense breasts,” Dr. Morris acknowledged. “We have investigated changes in our protocols to make this test easier, faster, and less expensive.”
Whereas the conventional MRI protocol involves a precontrast image and three postcontrast images, the abbreviated protocol involves one precontrast image and one postcontrast image (Figure 1). This focuses more on the “upslope of the dynamic kinetic curves” without much concern about the delayed imaging.
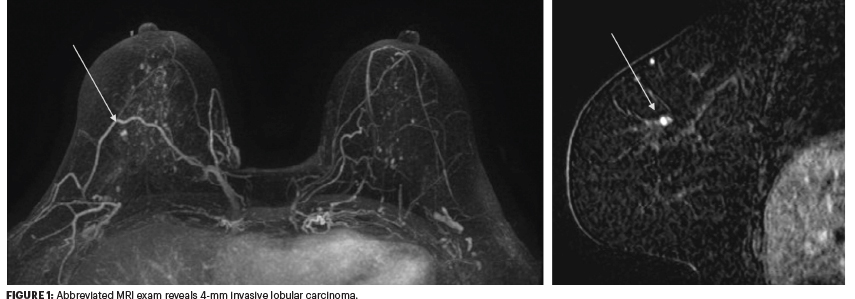
The next evolution is “ultra-fast” imaging, where contrast is seen entering the tumor with much greater clarity. The feeding vessel is also visible, making ultra-fast MRI a big leap compared with the older, steady-state type of imaging, she said.
Contrast-enhanced digital mammography is another less expensive alternative to MRI, though its uptake has been slow. This is an approach that incorporates the use of contrast to the standard screening mammogram, which Dr. Morris sees as a big improvement to current mammography.
Dr. Morris’ Recommendations for Screening
Dr. Morris summarized her recommendations for breast cancer screening based on patient risk:
Average Risk: Mammography (two-dimensional or three-dimensional) annually, starting at age 40; in addition, for patients with dense breasts, consider MRI.
Intermediate Risk: Mammography annually; consider MRI in patients with dense breasts and ultrasound in those with nondense breasts.
High Risk: Mammography beginning 10 years earlier than the youngest first-degree relative with breast cancer but not before age 25, with the addition of annual breast MRI.
A Fly in the Ointment
To address the problem of screening dense breasts, in September 2023 the U.S. Food and Drug Administration will begin requiring imaging facilities to give patients information about their breast density and to include specific language in its reports to explain how breast density can influence the accuracy of a mammogram. Additionally, facilities must recommend that patients with dense breasts discuss their individual risk with their health-care provider, including whether they need to consider any additional testing.
This mandate alone, however, may do little to facilitate the optimal screening of women with dense breasts. According to Dr. Morris, ultrasonography after mammography, MRIs, and even biopsies are frequently not covered by insurance, “which defeats the whole purpose,” she told The ASCO Post.
Imaging Surveillance After Treatment
Dr. Morris clarified some of the confusion regarding surveillance imaging after breast cancer treatment. Her recommendations in this area follow:
- After breast-conserving surgery, a first surveillance mammogram is typically performed at 6 months; thereafter, screening with standard mammography (not diagnostic) can be extended to a yearly exam.
- Mammography can be the standard type; diagnostic mammography is not necessary.
- MRI is recommended for women with dense breasts.
- After mastectomy, surveillance imaging is not generally recommended, not even for transverse rectus abdominis myocutaneous flaps or other types of autologous tissue transfer.
“There is really no need to do every-6-month follow-up for any certain length of time after successful treatment. The patient should be able to go back to regular screening,” she emphasized.
The uncertainty about surveillance imaging after breast cancer treatment is clear from a 2021 survey of radiologists.3 In the survey, 73% of radiology practices recommended diagnostic (not standard) mammography surveillance after breast-conserving therapy, with durations ranging from 6 months to 5 years before returning to regular screening mammograms. This was the common practice, despite the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) stating that “annual mammography” is recommended and not specifying that it be diagnostic or standard. The respondents said their protocols were based on the preferences of their referring clinicians and their own radiologists. The survey authors concluded there is “immense variability” in how the NCCN recommendations in clinical practice are handled and a lack of evidence-based literature on the type of mammography and surveillance intervals.
Imaging After Neoadjuvant Therapy
MRI is also the most accurate means of determining the magnitude of residual disease after neoadjuvant therapy and is preferred over positron-emission tomography (PET), mammography, and ultrasonography. Its efficacy was confirmed in the ACRIN 6657/I-SPY study,4 which showed that functional tumor volume was the most accurate way to predict response and progression-free survival—more accurate than changes in the longest diameter. Functional tumor volume combines functional criteria with a volume measurement, explained Dr. Morris.
The Future: Radiomics, AI
“Currently, what we are seeing on imaging and even on pathology is sort of a bird’s eye view of the cancer, but embedded in the 3D image is much more information that we could extract and analyze,” stated Dr. Morris. “One of the foundational aspects of deep learning is radiomics, a quantitative approach using spatial distribution of signal intensities and other imaging data not perceptible to the human eye. The rationale for radiomics is the assumption that electronic medical images contain information beyond visual perception…. We can take the pixels from the image and correlate them with the different pixels surrounding them.”
Numerous studies have shown that features produced by MRI-based radiomics may predict the possibility of achieving a pathologic complete response to neoadjuvant chemotherapy. “The image analysis in and of itself can improve performance,” she said.
For example, a Memorial Sloan Kettering Cancer Center team developed and validated a machine learning model that combines radiomics (using features seen before and after neoadjuvant therapy) with molecular subtype. The model accurately predicted pathologic complete response on MRI in an independent test set, with an area under the receiver operating curve of 0.88.5
Certainly, the future includes AI applications for cancer detection, risk determination, and post-treatment surveillance, she added. “The future in radiology is driving toward deep learning algorithms, where we use convolutional neural networks that basically mimic the brain’s neurons. And we don’t need a lot of human interaction with these algorithms. We just basically feed them lots of data, lots of MRI images of cancer undergoing preoperative chemotherapy, and it creates patterns within those images that can predict response,” Dr. Morris explained.
Indications are that the time has come for AI to be a stand-alone approach, at least for reading mammograms. “There are many companies showing that the AI-alone algorithm can outperform not only the individual radiologist, but the radiologist-plus-algorithm, so I think we have a very rich AI future,” she commented.
DISCLOSURE: Dr. Morris has served as a consultant or advisor to Bayer, Bracco, Guerbet Kheiron Medical, and Medscape and owns stock in Kheiron Medical and Reveal Pharma.
REFERENCES
1. Morris E: Practice patterns in the use of MRI in primary operable breast cancer. Invited Lecture. 2023 Miami Breast Cancer Conference. Presented March 3, 2023.
2. Bakker MF, de Lange SV, Pijnappel RM, et al: Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med 381:2091-2102, 2019.
3. Patel BK, Lee CS, Kosiorek HE, et al: Variability of postsurgical imaging surveillance of breast cancer patients: A nationwide survey study. AJR Am J Roentgenol 210:222-227, 2018.
4. Hylton NM, Blume JD, Bernreuter WK, et al: Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy—Results from ACRIN 6657/I-SPY trial. Radiology 263:663-672, 2012.
5. Sutton EJ, Onishi N, Fehr DA, et al: A machine learning model that classifies breast cancer pathologic complete response on MRI post-neoadjuvant chemotherapy. Breast Cancer Res 22:57, 2020.

