Over the past decade, an improved understanding of kidney cancer biology together with the development of novel systemic therapies have substantially improved the outcomes of patients with metastatic clear cell renal cell carcinoma (RCC).1 Following extensive clinical investigations, combinations of immune checkpoint inhibitors with or without vascular endothelial growth factor (VEGF) tyrosine kinase inhibitors currently represent a front-line standard of care in the management of patients with this disease.2,3 Despite such promising advances, many of these patients have tumors with intrinsic or acquired resistance to treatment, resulting in comparatively poor outcomes and almost universally lethal disease.2,3

Predictive biomarkers hold promise for addressing this challenge by helping clinicians to select the optimal therapy for their patients. However, the discovery of robust predictive biomarkers has been especially
challenging in metastatic clear cell RCC, where even validated biomarkers in other solid tumors, such as a high tumor mutational burden, are not associated with better outcomes in patients with this disease treated with immune checkpoint inhibitors.4-6 Genomic profiling of metastatic clear cell RCC similarly has not identified particular mutations consistently associated with a better response to immunotherapy, with the possible exception of PBRM1 loss-of-function mutations in the second-line setting following disease progression on VEGF tyrosine kinase inhibitors.7-9
More recently, RNA sequencing has emerged as a promising strategy to generate predictive biomarkers, as transcriptomic analyses of randomized clinical trials testing immunotherapeutic combinations in patients with metastatic clear cell RCC have identified molecular signatures associated with sensitivity or resistance to immune checkpoint inhibitors or tyrosine kinase inhibitors.5,6 These markers include the IMmotion150 T-effector, myeloid inflammation, and angiogenesis signatures,5 as well as the JAVELIN Renal 101 immunomodulatory and angiogenic transcriptomic profiles.6 Although these efforts have helped to better delineate some of the determinants of response to immunotherapy in clear cell RCC, these described signatures, in general, are not fully validated across different therapeutic regimens or clinical settings.6 For instance, the IMmotion 150 T-effector and myeloid inflammation signatures did not show an association with survival outcomes in both arms of the JAVELIN Renal 101 trial.6
IMmotion151 Key Findings
The IMmotion151 phase III randomized trial evaluated the efficacy of atezolizumab (an anti–PD-L1 inhibitory antibody) in combination with bevacizumab (an anti-VEGF monoclonal antibody), vs sunitinib (a VEGF tyrosine kinase inhibitor), in 915 patients with advanced RCC having a clear cell histology or sarcomatoid features.10 In the initial analysis, atezolizumab plus bevacizumab resulted in improved investigator-assessed progression-free survival, both in the intention-to-treat (ITT) and the PD-L1–positive populations (hazard ratio [HR] = 0.83, 95% confidence interval [CI] = 0.70–0.97; and HR = 0.74, 95% CI = 0.57–0.96, respectively).10 However, no significant difference for these outcomes was observed following radiologic central review.10 Additionally, there was no difference in overall survival in the ITT population between the two arms (HR = 0.93, 95% CI = 0.76–1.14).10
“Through molecular profiling, Motzer and colleagues have [identified] specific molecular subgroups of patients who may benefit from one therapeutic regimen or another.”— Toni K. Choueiri, MD, David A. Braun, MD, PhD, and colleagues
Tweet this quote
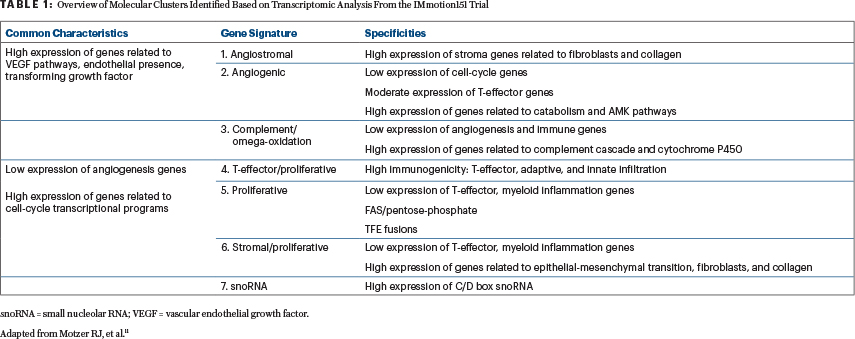
Whole-transcriptome analysis (through RNA sequencing) of 823 tumor samples from IMmotion151 followed by unsupervised clustering using nonnegative matrix factorization identified seven subgroups of patients with distinct molecular features (Table 1).11 Analysis of these molecular clusters in relation to progression-free survival identified improved outcomes with atezolizumab and bevacizumab in clusters 4 (T-effector/proliferative), 5 (proliferative), and 7 (snoRNA), and a trend toward a better response to sunitinib in clusters 1 (angiogenic/stromal) and 2 (angiogenic).11 Similar trends were also observed for these clusters in relation to the overall response rate.11
Final Overall Analysis
As summarized in this issue of The ASCO Post, the results of the final overall analysis from IMmotion151 were recently reported (at a minimum follow-up of 40 months) and continued to show no significant difference in overall survival in the ITT population between the two arms (HR = 0.91, 95% CI = 0.76–1.08).12 Although analysis of final overall survival results within each of the previously identified molecular clusters did not show any significant survival benefit for a particular therapeutic regimen,12 there was a trend toward better outcomes with atezolizumab plus bevacizumab in T-cell and proliferative clusters (4 and 5) and with sunitinib in an angiogenic cluster (2). Further, a significant survival benefit for the atezolizumab-plus-bevacizumab arm was observed when specific clusters were aggregated—grouping either clusters 4 and 5 (HR = 0.69,95% CI = 0.48–0.98) or clusters 4, 5, and 7 (HR = 0.70, 95% CI = 0.50–0.98).12
Through molecular profiling, Motzer and colleagues have dissected the clinical outcomes of patients with metastatic clear cell RCC from IMmotion151, identifying specific molecular subgroups of patients who may benefit from one therapeutic regimen or another. This further adds to the current body of evidence showing that molecular traits could help further stratify patients.
Unanswered Questions
Nevertheless, there remains a critical need to better define the utility of the recently described transcriptomic subgroups and their potential translation into clinical practice. Are these molecular clusters reproducible across different patient populations? Are these molecular subgroups predictive of response and survival with other immunotherapy combination regimens? What is the optimal therapeutic strategy for patients with metastatic clear cell RCC in a molecular cluster that does not show survival associations with any particular regimen (ie, clusters 3 [complement and omega-oxidation] and 6 [stromal/proliferative])? Moreover, some limitations are inherent to the statistical method used, as unsupervised clustering inevitably assigns each sample to the nearest cluster.13 One last element that should be considered while interpreting results is that the transcriptomic landscape varies between primary renal tumor and distant metastasis as well as between different metastatic sites.14 Assessing this heterogeneous landscape through a unified technique further supports the rationale for development of a potential liquid biomarker. Future translational studies will help provide a more concrete picture of the transcriptomic profiles across patients with metastatic clear cell RCC, along with a more accurate delineation of the therapeutic implications.
Clear cell RCC is a complex disease, characterized by an interplay of multiple molecular alterations and a heterogeneous microenvironment.4,15 The transcriptomic analyses from Motzer and colleagues represent a major step forward in molecularly classifying metastatic clear cell RCC, with the goal of identifying the determinants of response resistance in this disease. Moving forward, comprehensive investigations integrating multimodal data may further capture a more definitive picture of the disease, enabling a more accurate understanding and prediction of patient outcomes.
Dr. Saliby is a postdoctoral research fellow at Dana-Farber Cancer Institute. Dr. Labaki is Research Associate in Medicine at Dana-Farber Cancer Institute. Dr. McKay is Associate Professor of Medicine at Moores Cancer Center, University of California San Diego Health, La Jolla. Dr. Van Allen is Associate Professor of Medical Oncology at Dana-Farber Cancer Institute. Dr. Choueiri is Director of the Lank Center for Genitourinary Oncology and the Kidney Cancer Center at Dana-Farber Cancer Institute and the Jerome and Nancy Kohlberg Chair and Professor of Medicine at Harvard Medical School. Dr. Braun is Assistant Professor of Medicine (Medical Oncology) and Principal Investigator in the Center of Molecular and Cellular Oncology at Yale School of Medicine.
DISCLOSURE: Dr. Saliby reported no conflicts of interest. Dr. Labaki has received research funding from Roche/Genentech. Dr. McKay has served as a consultant or advisor to Aveo, Astellas, Medivation, AstraZeneca, Bayer, Bristol Myers Squibb, Calithera Biosciences, Caris, Dendreon, Exelixis, Janssen, Merck, Myovant, Novartis, Pfizer, Sanofi, Sorrento Therapeutics, Tempus, and Vividion Therapeutics. Dr. Van Allen has served as a consultant or advisor to Tango Therapeutics, Genome Medical, Genomic Life, Enara Bio, Janssen, Manifold Bio, and Monte Rosa; has received research support from Novartis and BMS; holds equity with Tango Therapeutics, Genome Medical, Genomic Life, Syapse, Enara Bio, Manifold Bio, Microsoft, and Monte Rosa; has institutional patents filed on chromatin mutations and immunotherapy response, and methods for clinical interpretation; intermittent legal consulting on patents for Foaley & Hoag; and serves on editorial boards for JCO Precision Oncology and Science Advances. Dr. Choueiri reported a financial and/or nonfinancial relationship with Alexion Pharmaceuticals, Alligent, Analysis Group, ASCO, AstraZeneca, Bayer, Bristol Myers Squibb, Cerulean Pharma, Clinical Care Options, Corvus Pharmaceuticals, Eisai, EMD Serono, Exelixis, Foundation Medicine, Genentech/Roche, GlaxoSmithKline, Harborside, Heron, Ipsen, Kidney Cancer Journal, The Lancet Oncology, Lilly, Lpath, Merck, Janssen, Michael J. Hennessy Associates, Navinata Healthcare, NCCN, The New England Journal of Medicine, Novartis, Peloton Therapeutics, Pfizer, PlatformQ Health, Prometheus, Sanofi/Aventis, and UpToDate. He has received institutional research funding from Agensys, Analysis Group, AstraZeneca, Bayer, Bristol Myers Squibb, Calithera Biosciences, Celldex, Cerulean Pharma, Congressionally Directed Medical Research Programs, Corvus Pharmaceuticals, Eisai, Exelixis, Foundation Medicine, Gateway for Cancer Research, GlaxoSmithKline, Ipsen, Merck, National Cancer Institute, Novartis, Peloton Therapeutics, Pfizer, Prometheus, Roche/Genentech, Seattle Genetics/Astellas, Takeda, and Tracon Pharma; holds international patents for biomarkers and has other relationships with ClinicalThinking, Envision Pharma Group, Fishawack Group of Companies, Health Interactions, and Parexel (medical writing assistance). He holds stock or other ownership interests in Pionyr Immunotherapeutics and Tempest Therapeutics. Dr. Braun has received honoraria from LM Education/Exchange Service; has served as a consultant or advisor to Adept Field Solutions, Blueprint Partnerships, Bristol Myers Squibb, Charles River Associates, Dedham Group, Defined Health, Insight Strategy, Octane Global, Schlesinger Associates, Slingshot Insights, Trinity Group, Aveo, Exelixis, and Catenion; and has been reimbursed for travel, accommodations, or other expenses by Bristol Myers Squibb.
REFERENCES
1. Braun DA, Bakouny Z, Hirsch L, et al: Beyond conventional immune-checkpoint inhibition: Novel immunotherapies for renal cell carcinoma. Nat Rev Clin Oncol 18:199-214, 2021.
2. Albiges L, Tannir NM, Burotto M, et al: Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 5:e001079, 2020.
3. Choueiri TK, Powles T, Burotto M, et al: Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384:829-841, 2021.
4. Braun DA, Hou Y, Bakouny Z, et al: Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat Med 26:909-918, 2020.
5. McDermott DF, Huseni MA, Atkins MB, et al: Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 24:749-757, 2018.
6. Motzer RJ, Robbins PB, Powles T, et al: Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: Biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat Med 26:1733-1741, 2020.
7. Braun DA, Ishii Y, Walsh AM, et al: Clinical validation of PBRM1 alterations as a marker of immune checkpoint inhibitor response in renal cell carcinoma. JAMA Oncol 5:1631-1633, 2019.
8. Conway J, Taylor-Weiner A, Braun D, et al: PBRM1 loss-of-function mutations and response to immune checkpoint blockade in clear cell renal cell carcinoma. medRxiv. November 4, 2020 (preprint).
9. Miao D, Margolis CA, Gao W, et al: Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 359:801-806, 2018.
10. Rini BI, Powles T, Atkins MB, et al: Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet 393:2404-2415, 2019.
11. Motzer RJ, Banchereau R, Hamidi H, et al: Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell 38:803-817.e4, 2020.
12. Motzer RJ, Powles T, Atkins MB, et al: Final overall survival and molecular analysis in IMmotion151, a phase 3 trial comparing atezolizumab plus bevacizumab vs sunitinib in patients with previously untreated metastatic renal cell carcinoma. JAMA Oncol 8:275-280, 2022.
13. Kiselev VY, Andrews TS, Hemberg M: Challenges in unsupervised clustering of single-cell RNA-seq data. Nat Rev Genet 20:273-282, 2019.
14. McKay RR, Barata PC, Elliott A, et al: Molecular alterations across sites of metastasis in patients with renal cell carcinoma. 2022 ASCO Genitourinary Cancers Symposium. Abstract 287.
15. Zhou M, Leung JY, Gessner KH, et al: PBRM1 inactivation promotes upregulation of human endogenous retroviruses in a HIF-dependent manner. Cancer Immunol Res 10:285-290, 2022.


