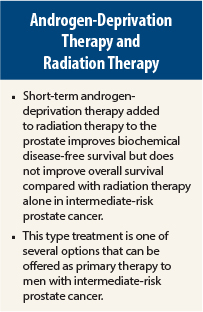
The addition of 6 months of androgen-deprivation therapy to radiation therapy as primary therapy for intermediate-risk prostate cancer improved biochemical control and disease-free survival but did not translate to an improvement in overall survival, in a phase III trial reported at the 2015 Genitourinary Cancers Symposium.1
Controversial Issue
“The role of short-term androgen-deprivation therapy in combination with radiotherapy for intermediate-risk prostate cancer has been controversial. Our study showed that short-term androgen-deprivation therapy added to radiation improved outcomes but did not improve overall survival,” said Abdenour Nabid, MD, a radiation oncologist at the Centre Hospitalier Universitaire de Sherbrooke, Quebec, Canada.
“Six months of short-term androgen deprivation therapy combined with radiotherapy represents one of several therapeutic options in intermediate-risk prostate cancer and should be discussed with patients treated for this type of cancer, taking into account age, comorbidities, erectile function, prognostic factors, and other parameters. It is important to consider the side effects of androgen-deprivation therapy and those of a higher dose of radiotherapy when discussing this with patients,” Dr. Nabid commented further.
The study had four endpoints. At the meeting, Dr. Nabid reported on the two primary endpoints (biochemical failure and disease-free survival) and a secondary endpoint (overall survival). The fourth endpoint is toxicity related to androgen-deprivation therapy and radiotherapy. All of these data are helpful in decision-making.
“These important data will soon be reviewed and will be reported next fall I hope,” he said.
Study Details
The study randomized 600 patients with intermediate-risk prostate cancer to one of three arms:
- Arm 1—short-term androgen-deprivation therapy plus radiation therapy to the prostate with 70 Gy over 7 weeks
- Arm 2—short-term androgen-deprivation therapy plus radiation therapy to the prostate with 76 Gy over 7.5 weeks
- Arm 3—prostate radiation therapy alone with 76 Gy over 7.5 weeks
Androgen-deprivation therapy was given for a total of 6 months: as neoadjuvant therapy for 4 months and as concomitant therapy with radiation therapy for 2 months.
The three arms were well balanced for baseline characteristics. The median age was 71, the median prostate-specific antigen (PSA) level was around 10 ng/mL, and the median Gleason score was 7. Overall, about 25% had stage T2b/c disease; 75% had a Gleason score of 7, and 57% had a PSA value of between 10 and 20 ng/mL.
Biochemical Control and Disease-Free Survival
Arm 2 had the lowest rate of biochemical failure. At 120 months, biochemical failure was observed in 23.4% of arm 1, 16.6% of arm 2, and 34.5% of arm 3. The number of metastases was significantly lower in arm 1 vs arm 3 (P = .011 for all metastases): node only 1% vs 1.5% respectively; bone metastases 0.5% vs 5%, respectively.
“This could mean that androgen-deprivation therapy prevents metastases,” Dr. Nabid suggested.
At a median follow-up of 75.4 months, disease-free survival was reported in 77.2% of arm 1 patients, 89.8% of arm 2 patients, and 64.7% of radiotherapy-alone patients (arm 2 vs arm 3 P < .001).
“Arm 1 and arm 2 are both better than arm 3 at 5 and 10 years. Arm 2 is the best for biochemical failure and disease-free survival,” he stated.
A total of 113 patients (19%) died, with only 6 deaths attributed to prostate cancer. The main cause of death was second cancers, with cardiovascular disease as the second cause of death. No significant overall survival difference was observed between the three arms at 5 and 10 years.
The study was conducted in the primary treatment setting. Dr. Nabid said, “We don’t know yet the answers about androgen-deprivation therapy and radiation in the salvage setting.” ■
Disclosure: Dr. Nabid has received a research grant from AstraZeneca, is on the advisory board of Sanofi/Janssen, is a speaker for Sanofi, and received financial support from Sanofi/AstraZeneca. The PCS III trial was funded by an AstraZeneca Pharmaceuticals grant.
Reference
1. Nabid A, Carrier N, Vigneault E, et al: Place of short-term androgen deprivation therapy in intermediate-risk prostate cancer treated with radiotherapy: A phase III trial. 2015 Genitourinary Cancers Symposium. Abstract 5. Presented February 26, 2015.