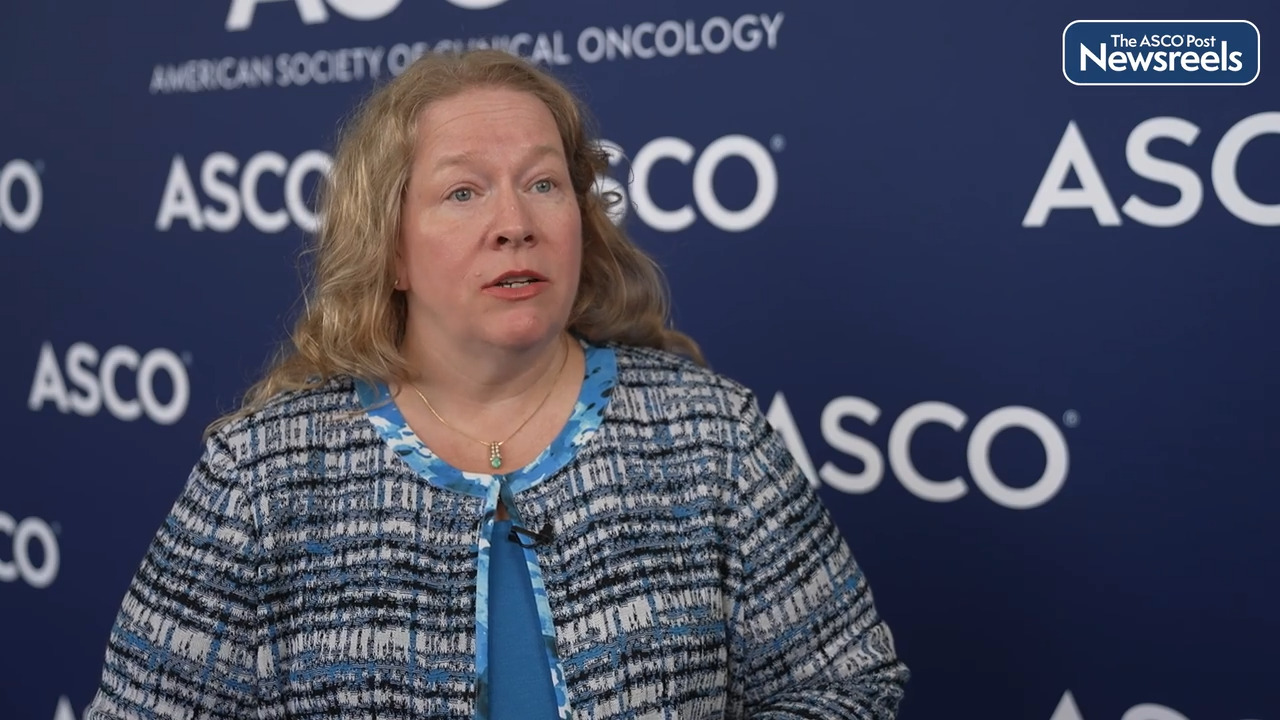Paula Aristizabal, MD, MAS, on Surviving Childhood Leukemia Near the Border of the United States and Mexico
2023 ASCO Annual Meeting
Paula Aristizabal, MD, MAS, of the University of California, San Diego, and Rady Children’s Hospital, talks about using a health systems strengthening approach to improve leukemia care and survival in a public Mexican hospital in the region of the border between the United States and Mexico. The demonstrated increase in overall survival across a decade after implementation of the program seems to validate the use of such models, not only to improve clinical outcomes, but also to build sustainable hospital capacity, financially and organizationally (Abstract 1502).
Transcript
Disclaimer: This video transcript has not been proofread or edited and may contain errors.
Paula Aristizabal, MD:
Acute lymphoblastic leukemia is the most common childhood cancer and survival has improved dramatically in high income countries to rates over 80%. Unfortunately, in low and middle income countries, survival has not increased at the same pace. For example, in Mexico, a low income country that is just across the border from the US, survival range is between 10% and 60%. Since in San Diego we share a border with Tijuana, we saw the disparities and we thought that it was our social responsibility to do something about it so we implemented a twinning program and twinning involves when a center of excellence in a high income country partners and collaborates with a center in a low, middle income country. We started this twinning program in 2008 in collaboration with St. Jude Children's Research Hospital, and we were able to implement a new team in Tijuana that was able to provide the best care possible for all the pediatric cancers.
Then in 2013, we realized that the burden of leukemia was getting higher and higher and we decided to implement a new model of health system strengthening called the WHO Framework for Action. The WHO Framework for Action has six building blocks that provide all the elements to improve care in a health system. We incorporated the WHO Framework for Action into the already existing twinning model to improve leukemia survival. We provided training to the team in Tijuana. We provided protocols that they could adapt. We provided mentorship and we provided support for a new infrastructure to develop the best possible leukemia care for children with with leukemia in Baja, California, Mexico.
After the implementation of the program, survival for leukemia improved from 59% to 65% and, importantly, the survival for standard risk leukemia improved from 73% before implementing the program to 100%, which is totally amazing because that is the same survival that we have in San Diego, just 20 miles from Tijuana. Survival for high risk leukemia improved from 48% to 55%. It wasn't so significant but we know that that is an area of improvement that we hope that we can tackle in our next steps of the program. Our model in Tijuana, combining a twinning program with the WHO Framework for Action, was effective in improving survival in a low and middle income country. This model can be applicable to a partnership between a high income country and a low, middle income country, especially in regions that share a border but also in other low and middle income countries remotely. Something that we learned from COVID is that you can apply many of these models working remotely and we are extremely pleased with these results. Our next steps include the improvement of the survival for patients with high risk leukemia.
Related Videos
The ASCO Post Staff
Arlene O. Siefker-Radtke, MD, of The University of Texas MD Anderson Cancer Center, discusses the combination of erdafitinib and cetrelimab, which demonstrated clinically meaningful activity and was well tolerated in cisplatin-ineligible patients with metastatic urothelial carcinoma and fibroblast growth factor receptor alterations (Abstract 4504).
The ASCO Post Staff
Jennifer A. Ligibel, MD, of Dana-Farber Cancer Institute, discusses a telephone-based weight loss intervention that induced clinically meaningful weight loss in patients with breast cancer who had overweight and obesity, across demographic and tumor factors. Additional tailoring of the intervention may possibly enhance weight loss in Black and younger patients as well (Abstract 12001).
The ASCO Post Staff
Allison Betof Warner, MD, PhD, of Stanford University Medical Center, and Zeynep Eroglu, MD, of H. Lee Moffitt Cancer Center and Research Institute, discusses phase II findings showing that in patients with BRAF-mutant metastatic melanoma, dabrafenib plus trametinib and navitoclax (DTN) was associated with a complete response rate of 20% and an overall response rate of 84%. Additionally, there was a trend toward improved overall survival in patients treated with DTN compared with dabrafenib plus trametinib alone; the difference in overall survival was more pronounced in patients with a smaller tumor burden (Abstract 9511).
The ASCO Post Staff
Tycel J. Phillips, MD, and Swetha Kambhampati, MD, both of City of Hope National Medical Center, discuss new findings showing that the real-world effectiveness and safety of brexucabtagene autoleucel were similar to data from the pivotal ZUMA-2 trial in patients with relapsed or refractory mantle cell lymphoma, regardless of prior BTK inhibition, bendamustine, or autologous stem cell transplantation (Abstract 7507).
The ASCO Post Staff
Tycel J. Phillips, MD, and Alex F. Herrera, MD, both of the City of Hope National Medical Center, discuss findings from the POLARIX study, which provided the largest prospectively collected circulating tumor DNA (ctDNA) data set on patients with previously untreated diffuse large B-cell lymphoma. Achieving ctDNA-negative status was associated with improved outcomes when patients were treated with polatuzumab vedotin-piiq plus combination chemotherapy vs combination chemotherapy alone (Abstract 7523).