Pearls in Neuro-oncology is guest edited by Tracy Batchelor, MD, Director, Division of Neuro-Oncology, Massachusetts General Hospital Cancer Center, and Professor of Neurology, Harvard Medical School, Boston. The series is intended to provide the practicing oncologist with guidance in managing neuro-oncology issues that may present in their patients with cancer.
Oncologists play a critical role in the interpretation of radiographic studies in glioblastoma patients, within an evolving therapeutic landscape. Integration of tumor molecular pathology, knowledge of radiation treatment plans, and an understanding of drug mechanisms of action are critical for accurate radiologic interpretation and patient management. One important issue that may arise in interpreting such images is the phenomenon of tumor “pseudoprogression”; oncologists need to be able to distinguish this effect from true disease progression.
Treating Glioblastoma
The standard of care for newly diagnosed glioblastoma patients consists of surgery followed by chemoradiation. Radiation is typically administered over 30 fractions (6 weeks) to a total tumor dose of 55 to 60 Gy in combination with daily temozolomide, an oral methylating agent, at 75 mg/m2/d. Subsequently, temozolomide (150–200 mg/m2) is administered 5 days each month for an additional 6 months. This approach is associated with superior progression-free and overall survival vs radiation alone.
A follow-up contrast-enhanced, cranial MRI is typically obtained 4 to 6 weeks from the conclusion of concurrent chemoradiation and prior to the start of additional chemotherapy. Interpretation of this first postchemoradiation MRI can be challenging. It is not uncommon to observe increased contrast-enhancement and surrounding T2/FLAIR (fluid-attenuated inversion recovery) hyperintensity within the radiation treatment field on this scan compared to the prechemoradiation scan. While these radiographic findings raise the possibility of tumor progression, they may also reflect the biologic effect of chemoradiation on the tumor and the tumor microenvironment, typically referred to as “treatment effect” or tumor “pseudoprogression.” However, development of new contrast enhancement outside the radiation treatment field represents tumor progression and should be treated accordingly.
Pseudoprogression Incidence and Findings
Tumor pseudoprogression was recognized in the era prior to the approval of temozolomide for glioblastoma. However, the incidence may have increased in the modern era of concurrent radiation and temozolomide, and a particular glioblastoma subtype may be more susceptible to tumor pseudoprogression.
The incidence of tumor pseudoprogression ranges from 28% to 66% in all glioblastoma patients undergoing chemoradiation and typically occurs within 3 months after the completion of concurrent radiation and temozolomide.1 Glioblastoma patients with promoter methylation of the repair enzyme 06-methyl guanine DNA methyltransferase (MGMT) may be at a higher risk of tumor pseudoprogression, with 91% (21 of 23 patients) of such patients developing early radiographic changes in one study.2
The radiographic findings typically consist of an increased area of contrast enhancement and enlargement of noncontrast T2/FLAIR hyperintense signal surrounding the enhancement. These radiographic changes range from mild to dramatic and may or may not result in neurologic symptoms. Unfortunately, other imaging techniques such as MRI-based perfusion and spectroscopy or brain FDG-PET scans cannot reliably distinguish tumor progression from pseudoprogression. Depending on the brain location or degree of mass effect, tumor pseudoprogression may result in exacerbation of existing neurologic symptoms and signs or the appearance of new impairments.
Management and Prognosis
Approximately one-third of patients are symptomatic from tumor pseudoprogression and may require treatment with corticosteroids. Bevacizumab (Avastin), a humanized, monoclonal antibody against the vascular endothelial growth factor (VEGF)-A ligand might be efficacious in the treatment of radiation-related brain necrosis but has not been adequately studied or established as an effective therapy for symptomatic tumor pseudoprogression.3 Although preliminary evidence suggests that concurrent treatment of newly diagnosed glioblastoma patients with chemoradiation and an inhibitor of VEGF may reduce the incidence of pseudoprogression, no VEGF inhibitor is yet approved for newly diagnosed glioblastoma, and follow-up studies are required.4
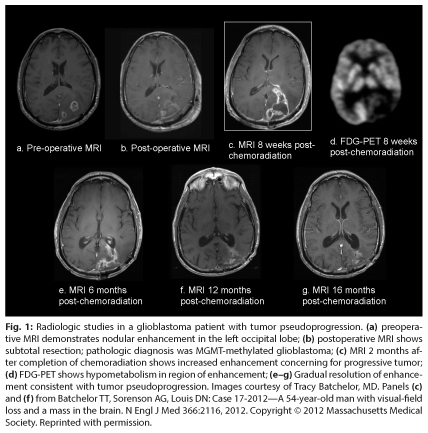
Asymptomatic patients with presumed pseudoprogression do not require any therapy, although short-interval brain MRI scans (every 6–8 weeks) are usually necessary to monitor the evolution of these changes and to exclude the possibility of tumor progression. Typically, the contrast enhancement and T2/FLAIR hyperintensity gradually diminish on serial MRI scans in patients with tumor pseudoprogression (Fig. 1). While tumor pseudoprogression may become symptomatic and require corticosteroid treatment, it appears to confer a favorable prognosis, with a median survival of 38 months in one report of 32 glioblastoma patients with established tumor pseudoprogression.
Clinical Trial Criteria
The recognition of tumor pseudoprogression in newly diagnosed glioblastoma patients has prompted a revision to radiographic response criteria and to eligibility criteria for clinical trials in this population.5 Patients with radiographic progression within 3 months of completion of concurrent chemoradiation are typically excluded from enrollment into an experimental trial unless the progression is outside the radiation treatment field or radiographic change within the radiation treatment field is confirmed as tumor progression by biopsy or resection.
Recommendations
In a glioblastoma patient who manifests increased contrast enhancement within the radiation treatment field on a cranial MRI scan within 3 months of completion of concurrent chemoradiation, I typically continue the standard, postradiation temozolomide course with follow-up MRI scans every 6 to 8 weeks. Corticosteroids are administered only if the radiographic changes result in new symptoms or an exacerbation of existing symptoms. If the contrast continues to expand at 6 months or beyond, a stereotactic biopsy is advised. If the contrast enhancement stabilizes or diminishes, a diagnosis of tumor pseudoprogression is likely and the patient should complete a standard course of temozolomide. ■
Disclosure: Dr. Batchelor is a consultant for Roche, Spectrum, Kirin Pharmaceuticals, Champions Biotechnology, and Advance Medical; receives research funding from Pfizer, AstraZeneca, Millennium, and National Institutes of Health; and receives CME lectures/contributions from Educational Concepts Group, Imedex, Robert Michael Educations Institute, UpToDate Inc., Research To Practice, and Oakstone Medical Publishing.
References
1. Fink J, Born D, Chamberlain MC: Pseudoprogression: Relevance with respect to treatment of high-grade gliomas. Curr Treat Options Oncol 12:240-252, 2011.
2. Brandes AA, Franceschi E, Tosoni A, et al: MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26:2192-2197, 2008.
3. Levin VA, Bidaut L, Hou P, et al: Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79:1487-1495, 2011.
4. Pinho MC, Polaskova P, Jennings D, et al: Impact of adjuvant anti-VEGF therapy on treatment-related pseudoprogression in patients with newly diagnosed glioblastoma receiving chemoradiation with or without anti-VEGF therapy. 2012 ASCO Annual Meeting. Abstract 2025. Presented June 1, 2012.
5. Wen PY, Macdonald DR, Reardon DA, et al: Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol 28: 1963-1972, 2010.