With exciting targeted and immunotherapeutic agents now part of the arsenal for metastatic melanoma, which drug should move to the head of the line? Mario Sznol, MD, Professor of Medicine at Yale University School of Medicine, New Haven, Connecticut, has been involved in key clinical trials of the new agents and was a co-leader of the phase I study of the anti–PD-1 monoclonal antibody nivolumab. Dr. Sznol shared his approach to treating advanced melanoma with attendees at the 3rd World Cutaneous Malignancies Congress in San Diego, and he elaborated on the topic in an interview with The ASCO Post.
Key Questions
What do you consider the key clinical questions on the minds of clinicians treating metastatic melanoma?
There are many. I will list some here:
If the patient has a BRAF V600E or V600K mutation, should I start with targeted therapy or immunotherapy, and does tumor volume or rate of progression matter in this setting?
- If a BRAF inhibitor is indicated, should I use vemurafenib (Zelboraf) alone, add the MEK inhibitor trametinib (Mekinist) to vemurafenib, or use the combination of dabrafenib (Tafinlar) plus trametinib? And if patients show progression on a BRAF inhibitor, should I add trametinib then?
- If I start with immunotherapy as a standard of care outside of a clinical trial, should I use high-dose interleukin-2 (Proleukin) or ipilimumab (Yervoy)?
- What is the future role of the anti–PD-1/PD-L1 antibodies, and how should I incorporate them in treatment?
- Is there still a role for chemotherapy?
Mutation Testing
How would you answer these questions for the oncologist? Let’s start with how you select initial treatment for the patient newly diagnosed with metastatic disease.
Although we consider immune therapy first in almost all patients, we will send a tumor sample for mutation testing, and the specifics of this depend on site of origin. If the primary disease was mucosal or acral lentiginous melanoma, we send off a sample for all the mutations (BRAF, NRAS, c-KIT) at once. If the disease originated from a cutaneous primary melanoma, we test for BRAF and NRAS. We add the test for c-KIT in some patients with primary lesions from chronic, sun-damaged skin. For ocular melanoma, we don’t always do genetic testing to determine therapy, although these tumors often have GNAQ or GNA11 mutations and may be somewhat responsive to MEK inhibitors.
If the patient does not have a BRAF V600E mutation, we consider that he or she could have other mutations or alterations in BRAF that are not picked up on the conventional test and that could respond to a BRAF or MEK inhibitor, so we may send tissue for more sophisticated molecular sequencing. Patients with the unusual mutations or alterations are a vanishingly small population, but that’s what personalized medicine is about. The testing algorithm will soon be changing, though, because multigene testing is becoming more affordable. Hospitals should soon have a panel of perhaps 500 to 1,000 genes.
Treatment Initiation
Based on the molecular profile, how do you initiate treatment? Do you start with a targeted agent or immunotherapy in BRAF-mutated patients?
The targeted agents are actually lower on our list, but it depends on the individual patient. While some patients can have durable responses to BRAF inhibitors, so far we don’t know if these responses are maintained once you stop the drug, unlike with immunotherapy.
If a BRAF inhibitor is indicated, the best recent data indicate it should be used in combination with the MEK inhibitor trametinib. The combined treatment is also better tolerated than a BRAF inhibitor alone, and we have had some success obtaining approval for the combination from insurance companies.
Even when we start targeted therapy first, our goal is to reach a level of response where we might stop the targeted therapy and start ipilimumab. We also have the option of going back to targeted therapy if there is no response to the immune therapy.
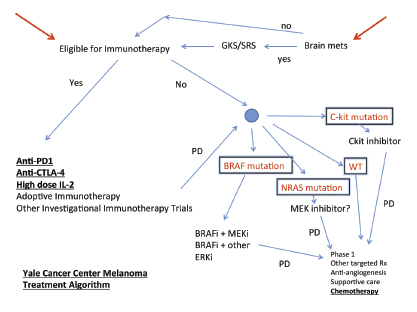
Our preferred approach is to start with a trial of immunotherapy (Fig. 1). We go through multiple lines of immunotherapy in an effort to induce a long-term remission, which may be a cure in some patients. If possible, we try to offer high-dose interleukin-2, ipilimumab, PD-1 blockade on a trial, or refer for a cell therapy trial, although not necessarily in that order.
For ipilimumab, it’s important to understand that some patients slowly build an immune antitumor response over 3 to 6 months, but once they respond they often have durable remissions—they just need time to get there. For patients with very rapid disease progression and for those with poor performance status, ipilimumab is probably not the drug to use first. However, agents such as anti–PD-1, alone or in combination with another agent, could give more rapid tumor responses, and therefore might be appropriate first choices in patients with more rapidly progressive disease or declining performance status.
Next Options
What do you do when the patient shows disease progression?
If the patient does not respond to immunotherapy and has a BRAF mutation, we move to the targeted agents—right now I prefer the combination of dabrafenib and trametinib. If the combination is not possible, we will offer vemurafenib or dabrafenib alone. If the patient has progression on vemurafenib, and he or she has had a long response to vemurafenib, we consider adding trametinib or changing to dabrafenib plus trametinib. If the patient has a c-KIT mutation, obviously we offer a drug like imatinib (Gleevec).
If disease progresses on all these treatments, we consider the patient for a phase I trial. The only role for chemotherapy at our center is in the patient who cannot tolerate, does not respond to, or is not a candidate to receive these newer agents.
It is really important to note that we still give priority to clinical trials at every decision point in the treatment process.
Novel Immunotherapeutic Agents
How will you use the anti–PD-1/PD-L1 antibodies?
We are very excited about these immunotherapeutic agents, currently nivolumab and lambrolizumab, which block PD-1 but also the antibodies that block PD-L1. About 30% of patients will respond to these agents by objective criteria, and conservatively, about half of this group may achieve very durable responses. The median response duration to nivolumab is 2 years—the longest we have seen in advanced melanoma.
Even better responses are observed when we combine nivolumab with ipilimumab. We see overall response rates of 40% to 50%, with about 30% of patients demonstrating at least an 80% tumor regression. This can occur even in patients with very large tumors that are progressing rapidly. The responses happen quickly and are very deep.
If you extrapolate from current data, and treatment starts with an anti–PD-1/PD-L1 agent, you can expect that 15% to 20% of your patients will have long, durable responses. With combinations, those durable responses may be achieved in as many as 25% to 30% of patients. In the future, that might, therefore, be your first treatment option in most cases.
With anti–PD-1, you can tell your patient that he or she will have a 30% chance of responding, and if a response occurs, there is a 50% chance that it will be durable, with almost no toxicity in most patients; toxicity, however, will be greater when PD-1 blockade is combined with ipilimumab. It’s a no-brainer that we will probably offer some form of PD-1 blockade first, alone or in combination.
Perhaps we may change this view when we see long-term data from the dabrafenib/trametinib or other BRAF/MEK targeted agent combinations. Of course, we need randomized trials of these combinations. I still don’t know with 100% certainty if nivolumab/ipilimumab is better than nivolumab alone, or better than lambrolizumab alone, or if one anti–PD-1/PD-L1 agent is better than another. But the impression is that for large-bulk, rapidly progressive disease, the combination may be capable of producing impressive responses within a period of weeks in some patients.
We believe that having these new drugs will make some of the current treatment algorithms obsolete, and in fact, the field is moving so rapidly that it is hard to predict the optimal algorithm for the next several years. ■
Disclosure: Dr. Sznol reported no potential conflicts of interest.