Subcutaneous implants containing testosterone in combination with a low dose of anastrozole can relieve menopausal symptoms in breast cancer survivors, according to research presented at the 2014 ASCO Breast Cancer Symposium.1
“Menopausal symptoms can be quite severe in breast cancer survivors in whom estrogen therapy is contraindicated. These women have been told that nothing can be done about this, and they are miserable. Our approach takes care of these symptoms—the joint pain and muscle aches, fatigue, even memory complaints,” said Rebecca Glaser, MD, a surgeon at Wright State University in Dayton, Ohio.
Since 2006, Dr. Glaser has performed more than 1,000 pellet insertions in patients with breast cancer using subcutaneous implants containing testosterone or testosterone plus anastrozole (Fig. 1). Many of her surgical patients continue seeing her for these implants. Oncologists also refer patients to her, and some postmenopausal women without cancer also seek her out for relief of menopausal symptoms, she told The ASCO Post.
The objective of the approach is to relieve symptoms related to hormone deficiency and/or endocrine therapy. “We are delivering testosterone and anastrozole for symptom relief. By the [internal review board] protocol, we are not treating their breast cancer, however, we believe there is an anticancer effect as well. We have seen no recurrences of cancer in up to 8 years of therapy,” Dr. Glaser said.
Study Details
The goal of the current study was to prospectively assess the clinical effect of this combination on menopausal symptoms—psychological, somatic, and urogenital complaints—in breast cancer survivors (stage 0–IV). Efficacy of therapy was documented using the validated, self-administered, 11-item Menopause Rating Scale (see sidebar), which patients completed prior to and following therapy. The study enrolled and analyzed 72 breast cancer survivors, most of whom were no longer being treated for cancer.
More than 90% of patients were treated with 8 mg anastrozole. The mean dose of testosterone, which is dosed based on weight, was 169 mg released over approximately 100 days. The pellets are completely absorbable and were inserted subcutaneously in the gluteal or inguinal area every 3 months on average. Therapeutic testosterone levels were confirmed without elevation of estradiol in any postmenopausal woman.
Reduction in Symptoms Attributed to Implant
The investigators noted statistically significant improvements in the Menopause Rating Scale total score and all subscales (P < .0001), including the following:
- Psychological complaints (depression, irritability/aggression, anxiety)
- Somatic complaints (hot flashes/sweating, heart discomfort, sleep problems, physical exhaustion, impaired memory, joint/muscular pain)
- Urogenital complaints (vaginal dryness, bladder problems/incontinence, sexual problems)
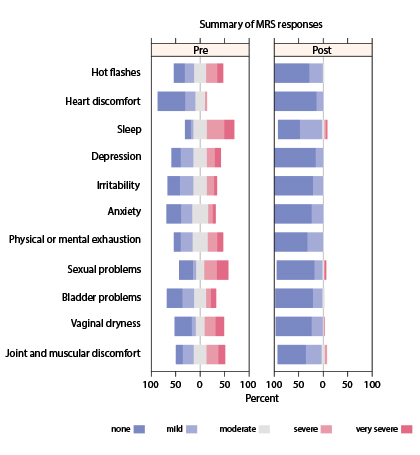
In addition, all individual symptoms or conditions, including hot flashes, improved after patients received the testosterone/anastrozole implants (P < .0001) (Fig. 2).
Total symptoms scores were reduced (ie, improved) by 81% in the total scale, by 86% on the psychological symptom scale, by 74% on the somatic symptom scale, and by 81% on the urogenital symptom scale.
“There have been no adverse drug events in any breast cancer survivor treated with parenteral testosterone/anastrozole therapy,” Dr. Glaser added. “The only side effect has been a slight increase in facial hair, and we offer laser hair removal for $25 for women bothered by this,” she added.
Rationale for the Treatment
There is strong rationale for using testosterone to treat symptoms related to hormone deficiency in women, she said. Testosterone is the most abundant biologically active female hormone (20 times that of estrogen), is essential for physical and mental health in women and is breast-protective, according to Dr. Glaser.
The women achieved a mean level of testosterone of 354 ng/dl, which may appear high, but testosterone’s effect is dose-dependent and these doses are necessary for the treatment to work, she pointed out.
“We have previously demonstrated that pharmacologic doses of exogenous testosterone delivered by the subcutaneous implant are necessary for a physiologic effect, replacing both testosterone and the significant contribution of androgen precursors to testosterone production at the cellular level,” she explained in interview.
“These patients are not 20-year-olds, who have high levels of testosterone and more importantly, thousands times higher levels of the androgen precursors DHEA [dehydroepiandrosterone] and androstenedione, which also decline with age,” she continued.
“Serum levels are fluctuating and transient. The bottom line is, you have to get enough testosterone to the androgen receptor to create a biological effect or clinical response. Some studies have failed to show efficacy because the doses were too low. The doses used in the study and levels on therapy are justifiable: we showed treatment efficacy—every symptom category improved significantly—as well as tolerability and safety,” she noted.
No elevations in estradiol have been observed in postmenopausal patients, she added.
“Our findings support testosterone’s direct, therapeutic effect via the androgen receptor,” Dr. Glaser offered. “Also, the absence of adverse events and the lack of breast cancer recurrences support the safety of these doses in breast cancer survivors.”
Alliance to Evaluate Approach
Dr. Glaser indicated that the Alliance A221102 Cooperative Group trial will evaluate the use of subcutaneous testosterone implants in the adjuvant treatment of postmenopausal women with arthralgias related to aromatase inhibitor use. The study will randomize 224 women to subcutaneous testosterone or placebo given every 3 months for 6 months. ■
Disclosure: Dr. Glaser reported no potential conflicts of interest.
Reference
1. Glaser RL, York AE, Dimitrakakis C: Breast Cancer Symposium. Abstract 109. Presented September 4, 2014.