The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) patient-level meta-analyses, concurrently reported in The Lancet, sought to clarify the effects of adjuvant aromatase inhibitor vs tamoxifen treatment and adjuvant bisphosphonate treatment in early breast cancer.1,2
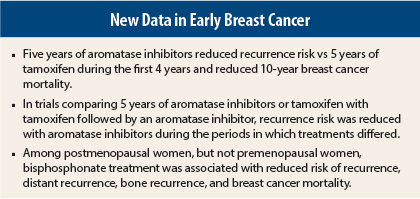
The endocrine treatment analysis indicated that 5 years of aromatase inhibitor treatment significantly reduced recurrence risk vs 5 years of tamoxifen therapy during the first 5 years and reduced 10-year breast cancer mortality vs tamoxifen.1 In trials where a comparator group received 2 to 3 years of tamoxifen followed by an aromatase inhibitor, recurrence rates were lower with aromatase inhibitors during the period that treatment differed between groups. The bisphosphonate analysis suggested a reduced risk of bone recurrence and breast cancer mortality, with the benefit limited to women who were postmenopausal at the start of treatment.2
Endocrine Therapy Study Details
The meta-analysis1 included individual data on 31,920 postmenopausal women with estrogen receptor–positive early breast cancer in randomized trials evaluating 5 years of an aromatase inhibitor vs 5 years of tamoxifen, 5 years of an aromatase inhibitor vs 2 to 3 years of tamoxifen then an aromatase inhibitor to year 5, and 2 to 3 years of tamoxifen then an aromatase inhibitor to year 5 vs 5 years of tamoxifen.
Outcomes were analyzed on an intent-to-treat basis stratified by age, nodal status, and trial, yielding aromatase inhibitor vs tamoxifen first-event rate ratios (RRs; P values are two-sided).
Comparison of Outcomes
In the comparison of 5 years of an aromatase inhibitor vs 5 years of tamoxifen, recurrence was significantly reduced with aromatase inhibitors during years 0 to 1 (RR = 0.64, 95% confidence interval [CI] = 0.52–0.78) and 2 to 4 (RR = 0.80, 95% CI = 0.68–0.93) and nonsignificantly thereafter. Ten-year breast cancer mortality was 12.1% vs 14.2% (RR = 0.85, P = .009).
In the comparison of 5 years of an aromatase inhibitor vs 2 to 3 years of tamoxifen followed by an aromatase inhibitor to year 5, recurrence was significantly reduced with aromatase inhibitors during years 0 to 1 (RR = 0.74, 95% CI = 0.62–0.89) but not while both groups received aromatase inhibitors during years 2 to 4 or thereafter. In these trials, risk of recurrence was lower with 5 years of aromatase inhibitors vs tamoxifen followed by aromatase inhibitors (RR = 0.90, P = .045), but the reduction in 10-year breast cancer mortality was not significant (RR = 0.89, P = .11).
In the comparison of 2 to 3 years of tamoxifen and then an aromatase inhibitor to year 5 vs 5 years of tamoxifen, risk of recurrence was significantly reduced with aromatase inhibitors during years 2 to 4 (RR = 0.56, 95% CI = 0.46–0.67) but not thereafter. Ten-year breast cancer mortality was lower with switching to aromatase inhibitors vs remaining on tamoxifen (8.7% vs 10.1%, P = .015).
When all three types of comparison were pooled, recurrence was significantly reduced with aromatase inhibitors during periods when treatments differed (RR = 0.70, 95% CI = 0.64–0.77) but not significantly thereafter (RR = 0.93, P = .08). Breast cancer mortality was reduced with aromatase inhibitors when treatments differed (RR = 0.79, 95% CI = 0.67–0.92), subsequently (RR = 0.89, 95% CI = 0.81–0.99), and for all periods combined (RR = 0.86, P = .0005). All-cause mortality was also reduced (RR = 0.88, P = .0003).
Aromatase inhibitor treatment was associated with reduced risk for endometrial cancers (10-year incidence = 0.4% vs 1.2%, RR = 0.33, 95% CI = 0.21–0.51) and increased risk of bone fractures (5-year risk = 8.2% vs 5.5%, RR = 1.42, 95% CI = 1.28–1.57).
The investigators concluded: “Aromatase inhibitors reduce recurrence rates by about 30% (proportionately) compared with tamoxifen while treatments differ, but not thereafter. Five years of an aromatase inhibitor reduces 10-year breast cancer mortality rates by about 15% compared with 5 years of tamoxifen, hence by about 40% (proportionately) compared with no endocrine treatment.”
Bisphosphonate Study Details
The meta-analysis included data on 18,766 women, including 18,206 in randomized trials of 2 to 5 years of bisphosphonate treatment, with a median follow-up of 5.6 woman-years, 3,453 first recurrences, and 2,106 subsequent deaths. Analysis used intention-to-treat log-rank methods to yield bisphosphonate group vs control group (open label or placebo, with no bisphosphonate) first-event rate ratios (P values are two-sided P values).
Reduced Risks in Postmenopausal Women
Overall, bisphosphonate treatment was associated with modest reductions in risk of recurrence (RR = 0.94, P = .08), distant recurrence (RR = 0.92, P = .03), and breast cancer mortality (RR = 0.91, P = .04) and a more definite reduction in risk for bone recurrence (RR = 0.83, P = .004).
There was no apparent effect of bisphosphonate treatment on any outcome among premenopausal women. Among 11,767 postmenopausal women, bisphosphonate treatment was associated with significant reductions in risk of recurrence (RR = 0.86, P = .002), distant recurrence (RR = 0.82, P = .0003), bone recurrence (RR = 0.72, P = .0002), and breast cancer mortality (RR = 0.82, P = .002).
Tests for heterogeneity of benefit in bone recurrence, the outcome with the greatest evidence of benefit, were significant by age (2P = .03) and menopausal status (2P = .06), but not bisphosphonate class, treatment schedule, estrogen receptor status, nodal status, tumor grade, or concomitant chemotherapy.
Bisphosphonate treatment had no effect on distant recurrence at extraosseus metastatic sites, locoregional recurrence, or the development of a new contralateral breast primary either overall or in the postmenopausal subgroup. No differences were observed in non–breast cancer mortality. Bisphosphonate treatment was associated with reduced risk of bone fractures
(RR = 0.85, P = .02).
The investigators concluded: “Adjuvant bisphosphonates reduce the rate of breast cancer recurrence in the bone and improve breast cancer survival, but there is definite benefit only in women who were postmenopausal when treatment began.” ■
Disclosure: Both studies were funded by Cancer Research UK, Medical Research Council. For full disclosures of the study authors, visit www.thelancet.com.
References
1. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG): Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet. July 23, 2015 (early release online).
2. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG): Adjuvant bisphosphonate treatment in early breast cancer: Meta-analyses of individual patient data from randomized trials. Lancet. July 23, 2015 (early release online).