
Stem cell transplant is associated with substantial morbidity and mortality, so our challenges are, first, to determine which patients are ideal candidates, and second, to know the opportune moment to proceed with stem cell transplant and the best means of doing so.— Ayalew Tefferi, MD
Tweet this quote
Allogeneic hematopoietic stem cell transplantation is the only potentially curative treatment for myelofibrosis. The ASCO Post asked an expert in this field, Ayalew Tefferi, MD, how and when he uses stem cell transplant in myelofibrosis, which is a topic he outlined in greater detail in the Journal of Oncology Practice.1 Dr. Tefferi is Professor of Medicine and Hematology at Mayo Medical School, Mayo Clinic, Rochester, Minnesota.
Principal Challenges
Hematopoietic stem cell transplantation is a potentially curative intervention for myelofibrosis. Is it widely used in this disease?
Allogeneic stem cell transplant is only feasible in younger and medically fit patients, and it is selectively offered as well to patients with high-risk disease. Despite many advancements, stem cell transplant is associated with substantial morbidity and mortality, so our challenges are, first, to determine which patients are ideal candidates, and, second, to know the opportune moment to proceed with stem cell transplant and the best means of doing so.
Patient/Disease Characteristics
What features would trigger a consideration for stem cell transplant?
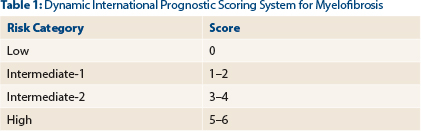
Myelofibrosis is primarily a disease of the elderly, and advanced age is associated with poor transplantation outcomes. The decision to perform hematopoietic stem cell transplantation depends on several patient-related variables, including age, performance status, and medical comorbidities, as well as disease biology. Our general recommendation is to routinely consider stem cell transplant in patients < 70 years old with a good performance status who have intermediate-2 or high-risk disease by the Dynamic International Prognostic Scoring System-plus (Table 1).
We also consider stem cell transplant in patients with low- or intermediate-1 Dynamic International Prognostic Scoring System-plus scores but with high-risk molecular features. We know that certain mutations have prognostic implications. Somatic mutations of JAK2, CALR, and MPL are responsible for the proliferative phenotype of myeloproliferative neoplasms, and they are usually referred to as “driver” mutations. Among them, type 1 or type 1-like CALR mutations are known to be associated with a favorable prognosis, and their presence in low/intermediate-1 risk disease argues against transplant for such patients. On the other hand, other mutations such as ASXL1 and SRSF2 portend poor overall or leukemia-free survival, and their presence suggests consideration of transplant sooner than later.
Impact of Donor Type
Can you tell us more about the differences in outcome based on the type of donor?
Most studies have shown that donor type is the most important factor influencing posttransplant survival. The ideal donor, according to most studies, is an human leukocyte antigen (HLA)-matched sibling, followed by a matched unrelated donor. Unmatched unrelated donors have the worst survival.
In a prospective study conducted by the Myeloproliferative Disorders Research Consortium, 2-year overall survival was 75% in the matched sibling group and 32% in the matched unrelated donor group.2 Similar distinctions can be seen for rates of graft failure, which a large study from the Center for International Blood and Marrow Transplant Research showed to be higher (20%) among patients with matched unrelated donors vs matched sibling donors (9%).3 Therefore, we believe that HLA-matched sibling or HLA-matched unrelated donors are the preferred options, and decisions to pursue unmatched donors should be individualized.
Alternative Graft Sources
Are there alternative graft sources that are being used clinically?
Peripheral blood and umbilical cord blood stem cells are being evaluated as graft sources, but these approaches remain controversial. Studies have been contradictory as to whether the use of peripheral blood stem cells results in more chronic graft-vs-host-disease and worse engraftment. Studies of umbilical cord blood transplant have been hampered by their retrospective nature and small sample sizes. At this point, we would limit the extent of these strategies.
Conditioning Regimens
What is the best conditioning regimen?
The choice of conditioning regimen remains unclear and should be based on patient age and comorbidity status. We generally aim for a myeloablative regimen in young and medically fit recipients. The largest study to date by the Center for International Blood and Marrow Transplant Research evaluated outcomes after myeloablative hematopoietic stem cell transplantation in 289 patients.3 The study showed 5-year overall survival rates to be 37% for HLA-identical sibling transplants and 30% for matched unrelated transplants; the 100-day treatment-related mortality rates were 18% and 35%, respectively. This study reinforced the curative potential as well as the toxicity of myeloablative conditioning stem cell transplant.
Score is based on five risk factors—age > 65 years, hemoglobin < 10 g/dL, white blood cell count > 25 x 109/L, peripheral blood blasts ≥ 1%, and constitutional symptoms—analyzed as time-dependent covariates in a multivariate Cox proportional hazard model. Risk factors were assigned score values based on hazard ratios. In the model-establishing study, estimated hazard ratios when shifting to the next risk category were 4.13 for low risk, 4.61 for intermediate-1, and 2.54 for intermediate-2. Using this model (or an age-adjusted version), prognosis can be assessed anytime during the course of primary myelofibrosis. Source: Passamonti F, et al: Blood 115:1703-1708, 2010.
However, myelofibrosis is usually diagnosed in elderly persons with comorbidities; therefore, reduced-intensity conditioning was developed. Fludarabine in combination with busulfan or melphalan or total-body irradiation is the most common strategy. In the largest retrospective series of patients (median age = 55) undergoing reduced-intensity preparative regimens, the 5-year overall survival was 56% for matched sibling donor transplants, 48% for matched unrelated donor donors, and 34% for mismatched unrelated donor transplant.4 These results reinforce the concept that reduced-intensity conditioning hematopoietic stem cell transplantation also has curative potential.
No prospective studies have compared myeloablative and reduced-intensity conditioning stem cell transplant in myelofibrosis, but retrospective studies suggest outcomes are similar. Due to the better toxicity profile, we believe the best choice for older patients and those with comorbidities is a reduced-intensity regimen.
Other Considerations
What other clinical issues are important to consider when referring patients to hematopoietic stem cell transplantation?
The optimal management of the spleen prior to stem cell transplant remains controversial. Data have been inconsistent as to whether splenectomy (for splenomegaly) confers a survival benefit, but we know that it can lead to complications. We therefore believe this decision should be individualized, but we consider it reasonable in patients with symptomatic massive splenomegaly. It is also reasonable to screen for asymptomatic portal hypertension in the pretransplantation workup, since numerous factors in myelofibrosis may impair liver function.
There is also the issue of managing relapses after stem cell transplant. In the absence of severe graft-vs-host disease, the tapering of immunosuppressive medication is a treatment option and can be followed by other strategies, including dose-escalated donor lymphocyte infusion and second transplantation.
Finally, with JAK inhibitors useful in myelofibrosis, their benefit in the transplant setting is being evaluated, but at this point they remain experimental. ■
Disclosure: Dr. Tefferi reported no potential conflicts of interest.
References

