Research reported at this year’s ASCO Annual Meeting shows major strides in treating ovarian and cervical cancers, suggesting the potential of new agents and adding evidence in areas where optimal management is unclear, according to Jonathan S. Berek, MD, of the Stanford Women’s Cancer Center, Stanford Cancer Institute, Stanford, California, who discussed these investigations at the Best of ASCO San Diego meeting.
Olaparib in Platinum-sensitive Ovarian Cancer
A randomized phase II trial tested olaparib, an oral inhibitor of poly-ADP-ribose polymerase (PARP), in platinum-sensitive recurrent serous ovarian cancer.1 A total of 162 patients were assigned to carboplatin/paclitaxel chemotherapy, with vs without olaparib during chemotherapy and also as maintenance.
With a median 5-month follow-up, the median progression-free survival was superior with olaparib vs without it (12.2 vs 9.6 months, P = .0012). Toxicity during the combination phase was generally similar between arms, and toxicity during olaparib maintenance was consistent with previous experience.
“This was a positive trial.… The toxicity overall was quite acceptable, certainly compared to what we usually use for either relapse treatment or maintenance therapy,” Dr. Berek commented.
“The group that did this study, which includes many of us, is doing a biomarker analysis, and we are pushing very hard to enable a larger, phase III trial, while we await the overall survival data,” he added. He also noted that there has been some reluctance to develop olaparib further in this disease (see sidebar, “Future of PARP Inhibitors in Ovarian Cancer Hangs in Balance”).
Bevacizumab in Platinum-resistant Ovarian Cancer
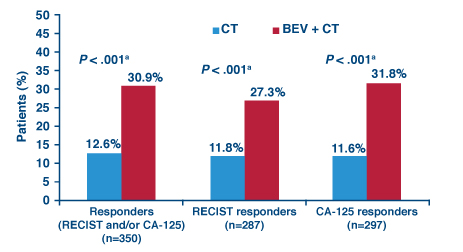
The randomized phase III AURELIA trial evaluated the addition of the antiangiogenic agent bevacizumab (Avastin) to chemotherapy in 361 patients with platinum-resistant recurrent ovarian cancer.2
With a median 18-month follow-up, progression-free survival was almost doubled with bevacizumab vs without it (6.7 vs 3.4 months, HR = 0.48, P < .001). Patients in the bevacizumab group were significantly more likely to have both radiographic and biochemical responses (Fig. 1).
Overall survival data are still immature. “A lot of these patients of course go on for even more treatment, and one can always be a bit concerned about what you do subsequently that might influence the overall survival data in any given study,” Dr. Berek cautioned.
The study was important in that it adds to data showing benefit of bevacizumab in the front-line setting and in the platinum-sensitive recurrence setting, although clear evidence of an overall survival benefit is still limited.
“It may be that bevacizumab over time will in fact be incorporated into both upfront maintenance and for the treatment of relapse,” he commented.
Dose-dense Paclitaxel in Ovarian Cancer
The randomized NOVEL trial (Japanese Gynecologic Oncology Group [JGOG] 3016) compared dose-dense weekly paclitaxel plus carboplatin vs conventional triweekly paclitaxel plus carboplatin in 631 patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer.3 Initial results, reported at a median follow-up of 29 months, had shown significantly better progression-free and overall survival with the dose-dense approach.
Updated results reported this year, now with a median follow-up of 6.4 years, still favored the dose-dense approach in terms of progression-free survival (28.2 vs 17.5 months) and overall survival (not reached vs 62.2 months). But in stratified analyses of overall survival, the dose-dense approach was significantly superior only among patients who had had residual disease measuring more than 1 cm (HR = 0.75) or serous histology (HR = 0.76).
“This is a very, very important finding, and probably the most important finding of the gynecologic cancer abstracts. This was a very impressive study, very well done,” Dr. Berek maintained. He noted that the gain in overall survival was similar to that seen with intraperitoneal regimens in other trials (see sidebar, “All Eyes on Trials of IV vs IP Chemotherapy for Ovarian Cancer”). “Ongoing trials compare this dose-dense regiment to intraperitoneal chemotherapy, and these comparisons will help to define how best to treat the majority of patients with these diseases,” he said.
Erlotinib Maintenance Falls Short in Ovarian Cancer
A randomized phase III cooperative group trial compared maintenance with erlotinib (Tarceva)—an epidermal growth factor receptor (EGFR) inhibitor—with observation in 835 patients with high-risk ovarian cancer who had no evidence of progression after first-line platinum-based chemotherapy.4
Fully 25% of patients in the erlotinib group stopped the drug because of adverse effects, mainly rash. With a median 26-month follow-up, erlotinib did not significantly improve median progression-free survival (12.7 vs 12.4 months) or overall survival (51 vs 59 months). Additional analyses failed to identify any subgroup of patients who derived benefit from the drug.
“Basically, this is a negative trial…This is in spite of the fact that we have pretty good phase I/II data that suggested that there should be some activity in this group of patients,” Dr. Berek commented.
“Why? Well it’s really not clear,” he continued. “Part of it, of course, is that EGFR-activating mutations are rare in ovarian cancer, and that may have something to do with why this is not a useful target.”
Carboplatin Is Alternate Partner to Paclitaxel in Advanced Cervical Cancer
The Japan Clinical Oncology Group (JCOG) 0505 randomized phase III trial pitted carboplatin plus paclitaxel against cisplatin plus paclitaxel in patients with stage IVB, persistent or recurrent cervical cancer.5
Among the 253 patients studied, carboplatin/paclitaxel was not inferior to cisplatin/paclitaxel in terms of median progression-free survival (6.2 vs 6.9 months, Pnoninferiority = .053) and overall survival (17.5 vs 18.3 months, Pnoninferiority = .032).
The rate of some adverse effects—neutropenia, and nausea and vomiting—and the percentage of days spent hospitalized were lower with carboplatin/paclitaxel.
“So can we recommend based on this that [carboplatin/paclitaxel] should be the new standard; is this conclusion justified? Well it appears to be a valid alternative,” at least for patients who have had prior cisplatin, as shown in subgroup analyses, Dr. Berek said. “It may be that the overall survival is not quite as good in cisplatin-naive patients, but that’s not clear from these data,” he noted.
“It’s basically balancing the side effects of one regimen to the other. We didn’t have in this study a good quality-of-life piggyback, which is really kind of frustrating, but I think because we are not going to cure this group of patients, you have to just be cautious about using a lot of cisplatin with them,” he concluded. “My personal preference is to switch to carboplatin…I have kind of been doing that for the last few years anyway, and now I just feel more comfortable about it based on this report.” ■
Disclosure: Dr. Berek receives research funding from AstraZeneca and Genentech.
References
1. Amit M. Oza, David Cibula, Ana Oaknin, et al: Olaparib plus paclitaxel plus carboplatin (P/C) followed by olaparib maintenance treatment in patients (pts) with platinum-sensitive recurrent serous ovarian cancer (PSR SOC): A randomized, open-label phase II study. 2012 ASCO Annual Meeting. Abstract 5001. Presented June 2, 2012.
2. Pujade-Lauraine E, Hilpert F, Weber B, et al: AURELIA: A randomized phase III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)-resistant recurrent ovarian cancer (OC). 2012 ASCO Annual Meeting. Abstract LBA5002. Presented June 2, 2012.
3. Katsumata N, Yasuda M, Isonishi S, et al: Long-term follow-up of a randomized trial comparing conventional paclitaxel and carboplatin with dose-dense weekly paclitaxel and carboplatin in women with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer: JGOG 3016 trial. 2012 ASCO Annual Meeting. Abstract 5003. Presented June 2, 2012.
4. Vergote IB, Joly F, Katsaros D, et al: Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: A GCIG and EORTC-GCG study. 2012 ASCO Annual Meeting. Abstract LBA5000. Presented June 2, 2012.
5. Kitagawa R, Katsumata N, Shibata T, et al: A randomized, phase III trial of paclitaxel plus carboplatin (TC) versus paclitaxel plus cisplatin (TP) in stage IVb, persistent or recurrent cervical cancer: Japan Clinical Oncology Group study (JCOG0505). 2012 ASCO Annual Meeting. Abstract 5006. Presented June 2, 2012.