 Designer T cells are modified from normal T cells to express specific immune receptors that allow them, via antibody-directed recognition or other mechanisms, to kill malignant cells bearing particular antigens. The Surgical Immunotherapy Lab at the Roger Williams Medical Center, Providence, Rhode Island, is focused on enhancing designer T-cell therapy for liver metastases. In a recent experimental study, investigators there identified a factor that suppressed the designer T-cell response to CEA-positive liver metastases and showed that the suppressive effect could be mitigated with regional infusion of anti-CEA designer T cells. The experimental model is designed to parallel the researchers’ ongoing Hepatic Immunotherapy for Metastases (HITM) phase I trial for patients with CEA-positive liver metastases (NCT01373047), in which patients receive percutaneous hepatic artery designer T-cell infusions.
Designer T cells are modified from normal T cells to express specific immune receptors that allow them, via antibody-directed recognition or other mechanisms, to kill malignant cells bearing particular antigens. The Surgical Immunotherapy Lab at the Roger Williams Medical Center, Providence, Rhode Island, is focused on enhancing designer T-cell therapy for liver metastases. In a recent experimental study, investigators there identified a factor that suppressed the designer T-cell response to CEA-positive liver metastases and showed that the suppressive effect could be mitigated with regional infusion of anti-CEA designer T cells. The experimental model is designed to parallel the researchers’ ongoing Hepatic Immunotherapy for Metastases (HITM) phase I trial for patients with CEA-positive liver metastases (NCT01373047), in which patients receive percutaneous hepatic artery designer T-cell infusions.
Steven C. Katz, MD, FACS, the principal investigator for the laboratory project and phase I trial, presented results of the research at a plenary session of the Society of Surgical Oncology Annual Symposium.
Study Design
 In the study, Katz and colleagues transduced murine splenocytes with a chimeric immune receptor containing an anti-CEA immunoglobulin moiety, the CD3ζ chain, and the CD28 costimulatory molecule. In vitro, designer T-cell proliferation was measured by flow cytometry, and myeloid-derived suppressor cells were added to assess their capacity to inhibit designer T-cell proliferation. CEA-positive colorectal liver metastases were established in mice via splenic injection, and designer T cells were infused via either the tail vein or the portal vein, with or without myeloid-derived suppressor cell depletion.
In the study, Katz and colleagues transduced murine splenocytes with a chimeric immune receptor containing an anti-CEA immunoglobulin moiety, the CD3ζ chain, and the CD28 costimulatory molecule. In vitro, designer T-cell proliferation was measured by flow cytometry, and myeloid-derived suppressor cells were added to assess their capacity to inhibit designer T-cell proliferation. CEA-positive colorectal liver metastases were established in mice via splenic injection, and designer T cells were infused via either the tail vein or the portal vein, with or without myeloid-derived suppressor cell depletion.
After injection of murine colorectal cancer cells, myeloid-derived suppressor cells expanded 2.4-fold compared with levels in normal mouse livers. Following 7 days of tumor growth, myeloid-derived suppressor cells accounted for 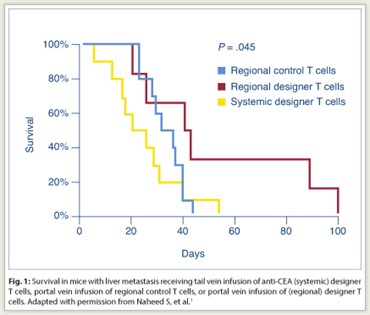 72.2% of liver CD45-positive cells, compared with 30.1% of cells in normal livers (P = .007). The addition of myeloid-derived suppressor cells to designer T cells resulted in a significantly decreased percentage of CD3-positive designer T cells that were undergoing division (53.1 vs 94.1%, P < .001). The suppressive effect of myeloid-derived suppressor cells was also significant for both CD4-positive and CD8-positive designer T cells (P < .001).
72.2% of liver CD45-positive cells, compared with 30.1% of cells in normal livers (P = .007). The addition of myeloid-derived suppressor cells to designer T cells resulted in a significantly decreased percentage of CD3-positive designer T cells that were undergoing division (53.1 vs 94.1%, P < .001). The suppressive effect of myeloid-derived suppressor cells was also significant for both CD4-positive and CD8-positive designer T cells (P < .001).
Portal vein infusion of designer T cells was performed to increase delivery to the liver and to determine whether such regional administration could reduce the suppressive effect of myeloid-derived suppressor cells in the liver. Portal vein injection was associated with a 6.7-fold increase in designer T-cell delivery to the liver compared with tail vein injection (P = .05). In mice with liver metastases, portal vein infusion of anti-CEA designer T cells was associated with a significant prolongation of survival compared with tail vein infusion (P = .045; Fig. 1). Animals received intraperitoneal anti-Gr1 antibody injections for in vivo myeloid-derived suppressor cell depletion. When myeloid-derived suppressor cell depletion was added to portal vein designer T-cell infusion, a significant increase in tumor cell death was noted by flow cytometry (P < .05).
Conclusions
As stated by Dr. Katz, “Growth of liver metastases results in a significant expansion of [myeloid-derived suppressor cells] that suppresses anti-CEA [designer T-cell] division. Regional infusion of [designer T cells] represents a potential strategy to overcome the suppressive intrahepatic milieu induced by liver metastasis growth, which we hope will be further enhanced through modulation of [myeloid-derived suppressor cells]. A multifaceted immunotherapeutic approach that targets both the tumor and host suppressive factors will be critical in order to achieve meaningful results for our patients with incurable metastatic disease.” ■
Disclosure: This work was supported by the Society of Surgical Oncology Clinical Investigator Award (supported by an educational donation provided by Genentech) and the Rhode Island Foundation.
Reference
1. Naheed S, Ahmed N, Nguyen C, et al: Society of Surgical Oncology 65th Annual Cancer Symposium. Abstract 2. Presented March 23, 2012.

