
ASCO’s recommendations and criteria to improve the development and implementation of oncology pathways serve as a first step in this path forward, leaving us filled with optimism for 2017 and beyond.— Robin T. Zon, MD, FACP, FASCO
Tweet this quote
The year 2016 was a memorable one for oncology. In January, President Barack Obama announced the launch of the National Cancer Moonshot initiative, spearheaded by Vice President Joe Biden, which aims to accelerate cancer research. And in December, through bipartisan Congressional support, the 21st Century Cures Act was signed into law, which provides significant funding to help make the goals of the Cancer Moonshot become a reality. There were other milestones in oncology care this past year as well, including the launch in June of the Centers for Medicare & Medicaid Services (CMS) Oncology Care Model, a physician-led specialty care program to provide higher quality, more coordinated oncology care at reduced costs. And in October, CMS released its final rule for implementation of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), a pay-for-performance system that emphasizes quality, not quantity, of care. All of these initiatives promise to alter the trajectory of cancer care for our patients.
The year 2016 was a pivotal one for ASCO as well. In June, Allen Lichter, MD, FASCO, stepped down as Chief Executive Officer of ASCO and was succeeded by long-time ASCO member and Past President of ASCO, Clifford A. Hudis, MD, FACP. It was also the year that saw expansion of CancerLinQ™, ASCO’s big data initiative, into over 50 vanguard practices nationwide and the publication of ASCO’s policy statement on clinical pathways in oncology,1 which includes recommendations to ensure that clinical pathways in oncology enhance—not diminish—patient care. Although these initiatives present inherent challenges, they also represent an opportunity to improve and preserve high-quality cancer care while addressing health care’s soaring costs. (See the March 2017 issue of the Journal of Oncology Practice for “The American Society of Clinical Oncology Criteria for High-Quality Clinical Pathways in Oncology.”2)
Improving Patient Care
The intent of ASCO’s policy statement was to elevate awareness about clinical pathways in oncology as an important tool for improving adherence to evidence-based medicine and reducing unwarranted variation in patient care and to convey a cautionary note that no current mechanism exists to ensure the integrity, efficient implementation, and outcome assessments for treatment management tools. The release came within a year of the establishment of ASCO’s Task Force on Clinical Pathways, which was charged with better understanding the concerns surrounding clinical pathway programs as articulated by ASCO members and other stakeholders.
Following the publication of the recommendations, the Task Force continued its efforts to ensure that pathways are consistently developed and transparent to all stakeholders as well as used in the way they are intended: to guarantee quality care while helping to reduce unwanted variations in care and control costs. As a result of ongoing deliberation and work, the Task Force developed a set of draft criteria for the development and implementation of high-quality oncology pathway programs while actively seeking and respecting input through direct stakeholder meetings and interviews with patient advocates, payers, vendors, and providers.
A set of 15 interrelated criteria developed by the Task Force, the “American Society of Clinical Oncology Criteria for High-Quality Clinical Pathways in Oncology,”3 released in November 2016, focus on 3 key areas: development, implementation/use, and analysis (Table 1). These criteria are intended to enable stakeholders to evaluate clinical pathway programs as well as guide future pathway development.
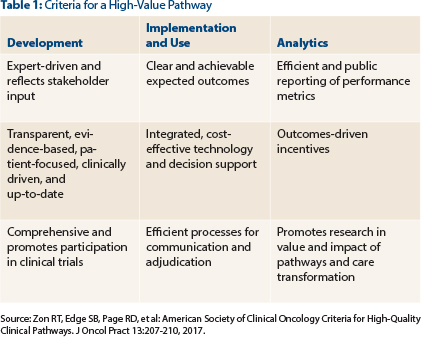
One could ask, however, do the published recommendations (Table 2) and criteria successfully address the concerns of our members? Consider this: Clinical pathways have been utilized for more than a decade in the oncology space, with the aim of improving patient care and communication, focusing on outcomes, maximizing resource utilization, reducing unwarranted variation, and promoting the use of higher value therapy. Yet the genesis of our work stemmed from a range of concerns articulated by members of ASCO’s State Affiliate Council and Clinical Practice Committee, including a lack of transparency in disclosing conflicts of interest and describing the methodology used in pathway development; a focus on cost savings, with efficacy and safety as secondary considerations; a cumbersome appeals process and no reimbursement for off-pathway treatment; no pathways for rare and inpatient-treated cancers; implementation requirements; and no publicized analytics supporting pathway utilization.

ASCO members especially highlighted the associated unsustainable administrative burdens related to the multitude of oncology pathways some providers are required to track and manage, as well as the continued requirement of preauthorization, even when pathway-compliant. To address these concerns, members of the Task Force developed the recommendations and criteria published in ASCO’s policy statement and in Criteria for High-Quality Pathways in Oncology. This work has proven to be the first venture into setting standards oncology pathway programs may want to consider implementing, and it may raise the bar for future pathway program development.
Although these criteria address many of the aforementioned concerns, the market response to pathway proliferation and alleviation of administrative burdens associated with pathway adherence has yet to be realized. In addition, meeting all the suggested criteria may require time and evolution of pathway program development, especially in the areas of preauthorization and meaningful analytic reporting.
As a consequence of the Task Force’s work, the magnitude and growing importance of pathways are evident, and deserving of our continued attention and ongoing efforts. It is hoped that this will further stimulate stakeholders to develop more integrated tools and processes for clinical pathway development, use, and analytics.
Transitioning to a Value-Based System
On another front, we can all appreciate that the primary goal of the statutory implementation of MACRA is to transition the health-care system away from a volume-incentivized, provider-centric model and toward a value-based, patient-centered model. Well-designed clinical pathways have the potential to help us adapt to this transformation. How? By serving as a foundation for comprehensive patient care while promoting efficient, targeted, and value-based care. This, in turn, can potentially help us control costs and better position practices to possibly assume financial risk for our patient populations while assuring their best care.
Ideally, pathways could potentially serve as a central component of oncology practice and as a cornerstone of future payment methodologies. Many oncology practices and health-care institutions have already adopted pathway programs, and some health insurance plans have implemented oncology-specific pathways.
Furthermore, ASCO’s high-quality pathway criteria can assist providers in evaluating pathway programs, as practices move to optimize controlling costs while preserving high-quality care. Consequently, a next step for the Task Force is to develop tools for evaluation of pathway programs.
Clinical pathway utilization may also intensify as various stakeholders collaborate to improve the delivery of patient care. Since cancer care is generally a multidisciplinary effort, pathways, if comprehensively developed as proposed in ASCO’s criteria statement, can be widely used by the caregiver team to optimally coordinate patient care. Reducing redundancy and unnecessary testing while assuring that necessary clinical evaluations and treatments are delivered is paramount to the success of a pathway program in this collaboration. More generally, collaboration between payers and providers regarding pathway utilization and criteria compliance may offer an opportunity to help minimize some of the administrative burdens for both stakeholder groups, provide leverage as a measure to control costs, and inform reimbursement discussions.
ASCO’s criteria for high-quality oncology pathway programs also promote a much-needed benefit of continually updated comprehensive pathways to assist with the management of rapidly developing clinical advances. Many oncologists care for patients with a variety of cancers and are challenged with implementing new advances that alter cancer care on a near-daily basis. All stakeholder groups, including payers and patients, are quick to point out variation in care and resource utilization among providers.
As pathways integrate scientific advances, including precision medicine and rapid learning system-validated evidence, there should be an opportunity to maximize resource alignment and promote value. Pathway programs can potentially assist providers in ensuring that the growing complexity of molecular testing is used optimally, so patients can receive targeted and other personalized care to achieve the best outcomes. It should be underscored that real-time incorporation of new information may serve as a tool to educate oncology providers as well as guide their decisions for patient care while simultaneously providing a mechanism to promote clinical research.
Containing Costs
Soaring health-care costs are being addressed in part in MACRA’s Quality Performance Program by requiring financial accountability of providers and patients. As a result, there is considerable concern for potential inappropriate rationing of care due to the resource utilization metric in the reward/penalty determination within the Quality Performance Program. State-of-the-art oncology interventions and therapies are costly, and the prices are not under the control of providers or patients. Consequently, although oncology providers are obligated to deliver appropriate, equitable care for all patients, they will be at high risk of penalty due to the associated high costs of oncologic care.
Many believe it is unfair to punish the provider for doing what is right for the patient. One consideration is to leverage pathway programs meeting the criteria to prove the delivery of high- quality care, which is appropriately cost-efficient while adding value, in the reward/penalty assessment rather than looking at cost alone. For this to be a viable option, there needs to be escalation of pathway program analysis as it pertains, firstmost, to patient outcomes and benefit, and, second, to the financial aspects surrounding cost and value for all stakeholders.
Looking Ahead
Pathway programs are likely here to stay, but they will continue to evolve. ASCO’s High Quality Criteria for Pathways stand as a catalyst for continued innovation toward fully integrated value- based care. The path forward is subject to managing the enhancements that pathways can bring to patient care while balancing the challenges to improve implementation and prove benefit at both the patient and societal levels.
ASCO’s recommendations and criteria to improve the development and implementation of oncology pathways serve as a first step in this path forward, leaving us filled with optimism for 2017 and beyond.
Acknowledgment: Dr. Zon would like to extend a special thanks to the significant and ongoing work of the current and past members of ASCO’s Task Force on Clinical Pathways, including Linda Bosserman, MD; Stephen Edge, MD; James Frame, MD; Gary Lyman, MD; Michael Neuss, MD; Jim Omel, MD; Ray Page, DO, PhD; and ASCO staff members Dana Wollins and Sybil Greene, JD. ■
Disclosure: Dr. Zon is Chair of ASCO’s Task Force on Clinical Pathways.
References
3. American Society of Clinical Oncology Criteria for High-Quality Clinical Pathways in Oncology. Available at www.asco.org/sites/new-www.asco.org/files/content-files/blog-release/documents/2016-ASCO-Criteria-High-Quality-Pathways.pdf. Accessed March 7, 2017.

