Multiple studies reported at the 2014 Gastrointestinal Cancers Symposium add further support for widening the genetic analysis of colorectal cancer tumors. In fact, experts predict that more extensive genetic testing for RAS gene mutations (in KRAS and NRAS) beyond the routine analysis of KRAS exon 2 will become the standard of care for identifying patients for treatments targeting the epidermal growth factor receptor (EGFR).
Predicting Response to Panitumumab
Marc Peeters, MD, PhD, Professor of Oncology at Antwerp University Hospital in Belgium, reported that activating mutations in less common exons of both KRAS and NRAS all predicted a lack of response to panitumumab (Vectibix) in the second-line setting.1 The findings corroborate recent findings from the PRIME and PEAK trials regarding the efficacy of panitumumab in first-line therapy, he said.
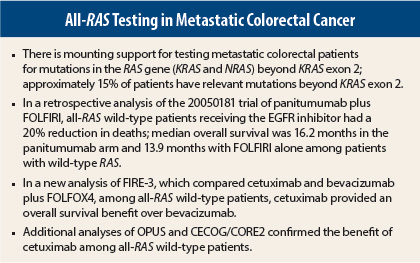
The study was a retrospective analysis from the randomized phase III 20050181 trial comparing the addition of panitumumab to second-line treatment with FOLFIRI (leucovorin/fluorouracil [5-FU]/irinotecan) in 1,186 patients. Investigators reanalyzed tumor specimens previously classified as wild-type KRAS exon 2, detecting additional mutations in exons 3 and 4 of KRAS and exons 2, 3, and 4 of NRAS. These new mutations occurred in 18% of patients with wild-type KRAS exon 2 disease and predicted a lack of response to the drug, Dr. Peeters reported.
Clinical outcomes were associated with these newly identified mutations. The overall survival hazard ratio (HR) of 0.850 (P = .12) in the previously defined KRAS wild-type population improved to 0.803 in the new analysis of all-RAS wild-type patients (P = .08). Median overall survival was 16.2 months in the panitumumab arm and 13.9 months with FOLFIRI alone. The progression-free survival hazard ratio improved from 0.73 (P = .004) to 0.695 (P = .006) when including all-RAS wild-type patients.
“If patients have a [RAS] mutant tumor, I don’t think there is a benefit to adding panitumumab to the chemotherapy backbone of FOLFIRI, but there is no deleterious effect,” he said. In the mutant RAS group, both arms had a median overall survival of approximately 11 months.
In the new analysis, the objective response rate to panitumumab/FOLFIRI also increased to 41% in the all-RAS wild-type patients, from 35% in the initial analysis.
“Based on all the data that’s been generated, it’s clear that today we need RAS testing instead of KRAS exon 2 testing before embarking on anti-EGFR treatment in patients with metastatic colorectal cancer,” he suggested.
Similar Findings With Cetuximab
The same conclusions were drawn with regard to cetuximab (Erbitux) in new analyses from three other studies: FIRE-3, OPUS, and CECOG/CORE2.
“Patients with all-RAS wild-type tumors have a clinically relevant survival benefit when first-line treatment with FOLFIRI plus cetuximab is offered,” said Sebastian Stintzing, MD, of the Klinikum Grosshadern and the Comprehensive Cancer Center at LMU Munich in Germany, who reported the FIRE-3 findings.2
The study compared cetuximab and bevacizumab (Avastin), added to FOLFIRI, as first-line treatment in 592 wild-type KRAS exon 2 metastatic colorectal cancer patients. New mutations were found in 16% of KRAS exon 2 wild-type patients, including KRAS exons 3 and 4 and NRAS exons 2, 3, and 4. Frequency of BRAF V600E mutation was 10%. PIK3CA mutations in exons 9 and 20 mutations were found in 7.3% and AKT mutations in 1%.
The median overall survival was significantly higher with cetuximab than bevacizumab in patients without RAS mutations, suggesting that the exclusion of patients with RAS mutations identifies a population more likely to benefit from cetuximab.
“No benefit was observed when patients with RAS-mutant tumors were treated with FOLFIRI plus cetuximab as compared to FOLFIRI plus bevacizumab,” Dr. Stintzing said.
An absolute overall survival benefit of 7.5 months was revealed when the analysis was done on the all-RAS wild-type population: Median overall survival was 33.1 months in the cetuximab arm and 25.6 months in the bevacizumab arm (HR = 0.70; P = .011). Interestingly, overall response rate and progression-free survival were not superior with cetuximab, and in the patients with RAS-mutant disease, bevacizumab actually led to better progression-free survival.
Though subsets of the more rare mutations were small, the study found comparable effects on overall survival for cetuximab and bevacizumab. Patients with BRAF mutations had a very poor progression-free and overall survival regardless of treatment designation. “The BRAF mutation is a very bad prognostic factor,” he noted.
Among PIK3CA-mutated patients, there was a trend toward longer median progression-free survival with bevacizumab (13.3 vs 7.8 months), but it was not statistically significant (P = .18). Median overall survival was about 26 months in each arm.
The findings from FIRE-3 and the 20050181 trial were upheld by mutational analyses from OPUS3 and the phase II CECOG/CORE2 trial,4 presented at the meeting.
Describing the OPUS analysis, Sabine Tejpar, MD, PhD, Associate Professor at the University Hospital Gasthuisberg in Leuven, Belgium, reported the breakdown of new RAS mutations beyond KRAS exon 2: KRAS exon 3 (6.8%), KRAS exon 4 (9.3%), NRAS exon 2 (7.6%), NRAS exon 3 (5.1%), and NRAS exon 4 (3.4%). Patients harboring any of these activating mutations were unlikely to benefit from cetuximab, she said.
“In the RAS wild-type population, there was significant benefit associated with the addition of cetuximab to FOLFOX4 [leucovorin/5-FU/oxaliplatin] in relation to progression-free survival and objective response rates, while the hazard ratio for overall survival also favored the FOLFOX4/cetuximab arm,” she said. “In the RAS mutant population, there was less favorable clinical outcome and no benefit—even harm—from the addition of cetuximab.”
Overall survival was approximately 4 months shorter in the cetuximab arm, among patients with RAS-mutant colorectal cancer, in the new OPUS analysis.
In the randomized phase II CECOG/CORE2 trial, investigators evaluated 148 KRAS exon 2 wild-type patients treated with first-line FOLFOX4 plus cetuximab and found that patients with RAS/BRAF wild-type disease had a significant prolongation of overall survival as compared to patients with mutant RAS/BRAF.
“This analysis supports the findings of other trials that RAS mutational analyses in metastatic colorectal disease is recommended prior to the initiation of an EGFR-targeted monoclonal antibody therapy,” said Thomas Brodowicz, MD, Associate Professor of Hematology/Oncology at the Medical University of Vienna and Central European Cooperative Oncology Group. n
Disclosure: Dr. Peeters is a consultant for and has received honoraria and research funding from Amgen. Dr. Stintzing is a consultant for Merck KGaA and Roche/Genentech and has received honoraria from Bristol-Myers Squibb, Merck Serono, and Roche/Genentech. Dr. Tejpar is a consultant for and has received honoraria from Merck Serono. Dr. Brodowicz has received honoraria from Amgen and Merck Serono. For full disclosures of all study authors, visit meetinglibrary.asco.org. ■
References
1. Peeters M, et al: Analysis of KRAS/NRAS mutations in phase 3 study 20050181 of panitumumab plus FOLFIRI versus FOLFIRI for second-line treatment of metastatic colorectal cancer. 2014 Gastrointestinal Cancers Symposium. Abstract LBA387. Presented January 18, 2014.
2. Stintzing S, et al: Mutations within the EGFR signaling pathway. 2014 Gastrointestinal Cancers Symposium. Abstract 445. Presented January 18, 2014.
3. Tejpar S, et al: Effect of KRAS and NRAS mutations on treatment outcomes in patients with metastatic colorectal cancer treated first-line with cetuximab plus FOLFOX4. 2014 Gastrointestinal Cancers Symposium. Abstract LBA444. Presented January 18, 2014.
4. Brodowicz T, et al: FOLFOX4 plus cetuximab administered weekly or every two weeks in first-line treatment of patients with KRAS and NRAS wild-type metastatic colorectal cancer. 2014 Gastrointestinal Cancers Symposium. Abstract LBA391. Presented January 18, 2014.