Two novel agents with distinct mechanisms of action join ranks of treatments that extend survival for patients with castration-resistant prostate cancer: MDV3100 and radium-223. Both drugs achieved a survival advantage compared with placebo, with relatively benign side-effect profiles, according to results of two international phase III trials reported at the 2012 Genitourinary Cancers Symposium, held recently in San Francisco.
 The first interim analysis of AFFIRM was presented by Howard I. Scher, MD, Memorial Sloan-Kettering Cancer Center, New York. Updated results of the effect of radium-223 on skeletal-related events in the ALSYMPCA trial were presented by Chris Parker, MD, Consultant Clinical Oncologist at the Royal Marsden Hospital, London. Survival results of ALSYMPCA were previously reported at the European Multidisciplinary Cancer Congress last fall (see The ASCO Post, October 15, 2011).
The first interim analysis of AFFIRM was presented by Howard I. Scher, MD, Memorial Sloan-Kettering Cancer Center, New York. Updated results of the effect of radium-223 on skeletal-related events in the ALSYMPCA trial were presented by Chris Parker, MD, Consultant Clinical Oncologist at the Royal Marsden Hospital, London. Survival results of ALSYMPCA were previously reported at the European Multidisciplinary Cancer Congress last fall (see The ASCO Post, October 15, 2011).
AFFIRM Analysis
“MDV3100 is a first-in-class androgen receptor signaling inhibitor. The oral investigational drug was rationally designed to inhibit androgen receptor signaling, which is a key driver of prostate growth,” Dr. Scher told listeners (Fig. 1).
At the first planned interim analysis of the randomized, placebo-controlled AFFIRM trial, MDV3100 reduced the risk of death by 37% in men with castration-resistant prostate cancer for whom docetaxel treatment had failed. At that time, an independent data monitoring committee recommended that the trial be halted and patients in the placebo 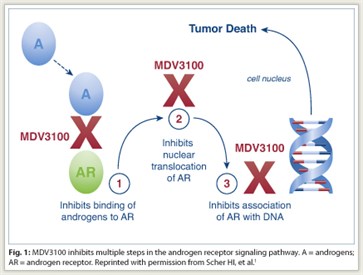 arm offered treatment with MDV3100.
arm offered treatment with MDV3100.
“The survival advantage coupled with the favorable side-effect profile positions MDV3100 as a potential front-line treatment in the postdocetaxel setting,” Dr. Scher said. The drug is currently under review at the FDA.
AFFIRM enrolled 1,199 men with castration-resistant prostate cancer in whom docetaxel treatment failed and randomly assigned them 2:1 to oral MDV3100 or matched placebo. Median overall survival was 18.4 months in the MDV3100 arm vs 13.6 months in the placebo arm (P < .0001). Median radiographic progression-free survival was also significantly superior for MDV3100 (8.3 vs 2.9 months; P < .0001). The survival benefit of MD3100 was significant across all prespecified subgroups.
Imaging and PSA
A significantly higher proportion of patients treated with MDV3100 showed tumor shrinkage on imaging (30% of patients had complete or partial responses to MDV3100 on imaging vs a 1.3% tumor shrinkage rate in the placebo group; P < .0001). In addition, a greater percentage of MDV3100-treated patients had at least a 50% or greater decline in PSA level (54% vs 1.5%; P < .0001).
“These favorable changes [in imaging and PSA] are consistent with the survival benefit,” Dr. Scher said.
The adverse event profile of MDV3100 was similar to that of placebo. More serious adverse events were reported in the placebo arm (38.6%) than in the MDV3100 arm (33.5%). More grade 3 or higher adverse events were also reported in the placebo arm (53.1% vs 45.3%). Adverse events of interest included a slightly higher percentage of patients with low-grade fatigue on the MDV3100 arm as well as a small number of seizures (0.6%). During the discussion following the presentation, Dr. Scher said that all five cases of seizures were reviewed thoroughly, and two of the cases were due to brain metastases. “The rare event of seizure is unlikely to deter physicians from prescribing MDV3100 given the robust survival benefit and the overal safety profile,” he commented.
Radium-223 is a first-in-class alpha-emitting radiopharmaceutical that mimics calcium and targets bone metastases with high-energy, very short-range alpha particles. These particles are more penetrant than gamma or beta particles. Radium-223 potentially spares healthy tissue, thereby limiting side effects.
ALSYMPCA enrolled 922 men with progressive, symptomatic metastatic castration-resistant prostate cancer with two or more known bone metastases but no visceral metastases. They were randomly assigned to radium-223 vs placebo in a 2:1 ratio.
As reported previously, radium-223 significantly improved overall survival compared with placebo in the ALSYMPCA trial. Median overall survival was 14 months for radium-223 vs 11.2 months for placebo (P = .00185). The trial was halted early due to the benefit in overall survival.
Skeletal-related Events
At the Genitourinary Cancers Symposium, Dr. Parker presented the first analysis of skeletal-related events. Median time to first skeletal-related event was significantly delayed in the radium-223 group (13.6 vs 8.4 months; P = .00046).
Looking at the individual components of skeletal-related events, radium-223 exerted a preventive effect on pathologic bone fracture (4% vs 7%; P = .013), spinal cord compression (3% vs 6%; P = .016), and need for external-beam radiation (23% vs 27%; P = .0038). No difference between treatment arms was seen in terms of need for surgical intervention (2% in both arms).
First to Prevent Spinal Cord Compression
“To our knowledge, radium-223 is the first drug ever shown to be effective in the prevention of spinal cord compression. This is an important observation, because this is a devastating complication of bone metastases,” Dr. Parker stated.
Safety and tolerability of radium-223 was extremely favorable, with a low incidence of myelosuppression: Incidence of grade 3/4 neutropenia was 1.8% for radium-223 vs 0.8% for placebo, and of grade 3/4 thrombocytopenia, 4% vs 2%, respectively. A lower percentage of patients in the radium-223 group reported adverse events: 88% vs 94% for adverse events of any grade, and 51% vs 59% reporting grade 3/4 adverse events. ■
Disclosure: Dr. Parker has received honoraria from Bayer for serving on advisory boards. Dr. Scher has been a consultant or advisor for and has received research funding from Medivation.
Expert Point of View: Two Novel Agents Prolong Survival in Advanced Prostate Cancer
References
1. Scher HI, Fizazi K, Saad F, et al: Effect of MDV3100, an androgen receptor signaling inhibitor (ARSI) on overall survival in patients with prostate cancer postdocetaxel: Results from the phase III AFFIRM study. 2012 Genitourinary Cancers Symposium. Abstract LBA1. Presented February 2, 2012.
2. Parker C, Heinrich D, O’Sullivan JM, et al: Overall survival benefit and safety profile of radium-223 chloride, a first-in-class alpha-pharmaceutical: Results from a phase III randomized trial (ALSYMPCA) in patients with castration-resistant prostate cancer (CRPC) with bone metastases. 2012 Genitourinary Cancers Symposium. Abstract 8. Presented February 2, 2012.


 “My comment on the AFFIRM trial is ‘wow, very impressive.’ The median survival and the declines in PSA levels are impressive, and this is going to change the way we care for patients in our practices,” said Nicholas Vogelzang, MD, medical oncologist with Comprehensive Cancer Centers of Nevada and...
“My comment on the AFFIRM trial is ‘wow, very impressive.’ The median survival and the declines in PSA levels are impressive, and this is going to change the way we care for patients in our practices,” said Nicholas Vogelzang, MD, medical oncologist with Comprehensive Cancer Centers of Nevada and...