ASCO recently convened an update committee of experts in medical oncology, pathology, radiation oncology, surgical oncology, guideline implementation, and advocacy to develop evidence-based recommendations that update the ASCO 2005 clinical practice guideline on use of sentinel node biopsy in patients with early-stage breast cancer.1
The update is provided for medical oncologists, radiation oncologists, pathologists, surgeons, oncology nurses, patients/caregivers, and guideline implementers. Gary H. Lyman, MD, MPH, FASCO, of Fred Hutchinson Cancer Research Center, University of Washington, and Armando E. Giuliano, MD, of the John Wayne Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, were the co-chairs of the update committee.
Methods
The update committee performed a systematic review of the literature from February 2004 through January 2013 to review new evidence bearing upon three primary clinical questions: (1) How should the results of sentinel node biopsy be used in clinical practice? (2) What is the role of sentinel node biopsy in special circumstances in clinical practice? (3) What are the potential benefits and harms associated with sentinel node biopsy?
Nine randomized clinical trials met the systematic review criteria for clinical questions 1 and 2, and 13 cohort studies provided evidence pertaining to clinical question 3. No phase II studies, meta-analyses, or other systematic reviews were identified that met the systematic review criteria for the guideline update.
Summary of Update
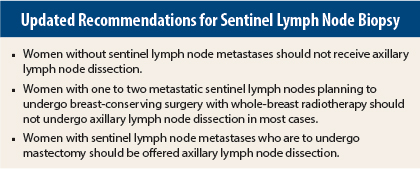
In brief, recommendations based on new information from randomized clinical trials include the following: women without sentinel lymph node metastases should not receive axillary lymph node dissection. Women with one to two metastatic sentinel lymph nodes planning to undergo breast-conserving surgery with whole-breast radiotherapy should not undergo axillary lymph node dissection (in most cases). Women with sentinel lymph node metastases who are to undergo mastectomy should be offered axillary lymph node dissection.
Recommendations based on new information from cohort studies or informal consensus are as follows: Women with operable breast cancer and multicentric tumors, with ductal carcinoma in situ who will undergo mastectomy, who previously underwent breast or axillary surgery, or who received preoperative/neoadjuvant systemic therapy may be offered sentinel node biopsy. Women who have large or locally advanced invasive breast cancer (T3/T4), inflammatory breast cancer, or ductal carcinoma in situ (when breast-conserving surgery is planned) or who are pregnant should not undergo sentinel node biopsy.
In some cases, updated evidence was not sufficient to warrant updating of previous recommendations.
Recommendations
The individual recommendations, type and strength of supporting evidence, and strength of recommendation are provided below.
Recommendation 1: Clinicians should not recommend axillary lymph node dissection for women with early-stage breast cancer who do not have nodal metastases. Type of recommendation = evidence-based, benefits outweigh harms. Evidence quality = high. Strength of recommendation = strong.
Recommendation 2.1: Clinicians should not recommend axillary lymph node dissection for women with early-stage breast cancer who have one or two sentinel lymph node metastases and are to receive breast-conserving surgery with conventionally fractionated whole-breast radiotherapy. Type of recommendation = evidence-based, benefits outweigh harms. Evidence quality = high. Strength of recommendation = strong.
Recommendation 2.2: Clinicians may offer axillary lymph node dissection to women with early-stage breast cancer with nodal metastases found on sentinel node biopsy who are to receive mastectomy. Type of recommendation = evidence-based, benefits outweigh harms. Evidence quality = low. Strength of recommendation = weak.
Recommendation 3: Clinicians may offer sentinel node biopsy to women who have operable breast cancer and who have the following characteristics/circumstances:
Recommendation 3.1: Multicentric tumors. Type of recommendation = evidence-based, benefits outweigh harms. Evidence quality = intermediate. Strength of recommendation = moderate.
Recommendation 3.2: Ductal carcinoma in situ when mastectomy is performed. Type of recommendation = informal consensus, benefits outweigh harms. Evidence quality = insufficient. Strength of recommendation = weak.
Recommendation 3.3: Prior breast and/or axillary surgery. Type of recommendation = evidence-based, benefits outweigh harms. Evidence quality = intermediate. Strength of recommendation = strong.
Recommendation 3.4: Preoperative/neoadjuvant systemic therapy. Type of recommendation = evidence-based, benefits outweigh harms. Evidence quality = intermediate. Strength of recommendation = moderate.
Recommendation 4: There are insufficient data to change the 2005 recommendation that clinicians should not perform sentinel node biopsy for women who have early-stage breast cancer and meet the following criteria:
Recommendation 4.1: Large or locally advanced invasive breast cancers (tumor size T3/T4). Type of recommendation = informal consensus. Evidence quality = insufficient. Strength of recommendation = weak.
Recommendation 4.2: Inflammatory breast cancer. Type of recommendation = informal consensus. Evidence quality = insufficient. Strength of recommendation = weak.
Recommendation 4.3: Ductal carcinoma in situ when breast-conserving surgery is planned. Type of recommendation = informal consensus. Evidence quality = insufficient. Strength of recommendation = strong.
Recommendation 4.4: Pregnancy. Type of recommendation = informal consensus. Evidence quality = insufficient. Strength of recommendation = weak.
Qualifying Statements
As a qualifying statement, the guideline update notes that clinicians may perform sentinel node biopsy for ductal carcinoma in situ diagnosed by minimally invasive breast biopsy under three conditions: (1) when mastectomy is planned, because this precludes subsequent sentinel node biopsy at a second operation; (2) when physical examination or imaging reveals a lesion highly suggestive of invasive cancer; and (3) when the area of ductal carcinoma in situ on imaging is large (≥ 5 cm). It is also noted that sentinel node biopsy may be offered before or after neoadjuvant systemic therapy, although results appear to be less accurate for sentinel node biopsy performed after neoadjuvant therapy.
The committee also notes that the current update deletes a 2005 recommendation regarding patients who had undergone prior nononcologic breast surgery or axillary surgery, due to insufficient data on which to base a recommendation.
A data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources are available at www.asco.org/guidelines/breastsnb. Patient information is available at www.cancer.net. ■
Disclosure: The Update Committee reported no potential conflicts of interest.
Reference
1. Lyman GH, Temin S, Edge SB, et al: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. March 24, 2014 (early release online).