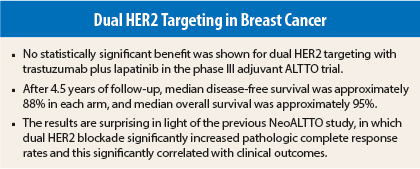
The highly anticipated results from the phase III ALTTO trial show no additional benefit for adding lapatinib (Tykerb) to trastuzumab (Herceptin) in the adjuvant treatment of HER2-positive breast cancer.1
The results were presented at the 2014 ASCO Annual Meeting’s Plenary Session by Martine J. Piccart-Gebhart, MD, PhD, Chair of the Breast International Group in Brussels, and at a media briefing by Edith A. Perez, MD, Deputy Director at Large at the Mayo Clinic Cancer Center, Jacksonville, Florida. Dr. Piccart-Gebhart and Dr. Perez are co–principal investigators of ALTTO.
“The study failed to show that lapatinib adds to the benefit of trastuzumab in terms of disease-free survival,” Dr. Perez reported prior to the plenary presentation. “At this time of follow-up, there is also no statistically significant difference in overall survival with dual [concomitant] blockade, or sequential anti-HER2 treatment, vs single-agent trastuzumab.”
As the discussant of this paper at the Plenary Session, George W. Sledge, Jr, MD, Professor of Medicine and Director of the Division of Oncology at Stanford University, and a Past President of ASCO, delivered some somber remarks. “This is a negative trial.… This is a serious disappointment,” he said (see Expert Point of View below).
But Dr. Perez did find something positive in the results, as she noted to the media that the 555 disease-free events that occurred at 4.5 years fell far short of the 850 that were anticipated in the statistical plan. “Another way of looking at this study is that the patients overall did pretty well—better than anticipated,” she said.
“In addition, we were very impressed by the very low rate (< 1%) of observed significant cardiac toxicity in all of the treatment arms, in the context of approximately 97% having received anthracyclines,” she told The ASCO Post.
Unexpected Results
Dr. Perez emphasized that failure to meet the primary endpoint—improved outcomes with dual HER2 blockade—was unexpected. “We were surprised that adding lapatinib did not provide further benefit, since the combination of these drugs was promising in a smaller study,” she said.
Dr. Perez was referring to the NeoALTTO trial, which evaluated the combination vs each single agent as neoadjuvant treatment in 455 patients. In NeoALTTO, dual HER2 blockade resulted in a near doubling of the pathologic complete response rate (51.3%), vs single-agent trastuzumab (29.5%) or lapatinib (24.7%); the reaching of pathologic complete response corresponded to a 62% reduction in events as compared with no pathologic complete response, Dr. Piccart-Gebhart reported at the 2013 San Antonio Breast Cancer Symposium.2
In her presentation at ASCO, Dr. Piccart-Gebhart noted that NeoALTTO was not alone: four out of six previous studies of dual blockade in the neoadjuvant setting—using mostly trastuzumab and lapatinib, but also, in the NeoSphere trial, trastuzumab and pertuzumb (Perjeta)—demonstrated benefit, as did two studies in the advanced-disease
setting.
The ALTTO (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) investigators hypothesized that in the adjuvant setting, two HER2-targeted agents would be superior to trastuzumab alone in preventing breast cancer recurrences. It was the largest-ever adjuvant clinical trial in HER2-positive breast cancer, involving 8,381 women from 946 centers in 44 countries, Dr. Piccart-Gebhart noted in her presentation.
A P value of ≤.025 was required for statistical significance in order to test both the concurrent and the sequential treatments, vs trastuzumab. The single-agent lapatinib arm was closed early due to futility, and the results of that arm will be presented later this year. The current efficacy results were based on 6,281 patients randomized.
ALTTO Schema
In the design, described as “pragmatic” by Dr. Piccart-Gebhart, patients were randomly assigned after surgery to the concurrent use of trastuzumab and lapatinib, to the sequential use of trastuzumab followed by lapatinib, or to trastuzumab alone for 1 year. The following dosing schemes were used:
- Design 1, after completion of chemotherapy: (1) trastuzumab at an 8 mg/kg intravenous loading dose, followed by 6 mg/kg intravenously every 3 weeks for 52 weeks; or (2) lapatinib at 1,500 mg/d for 52 weeks; or (3) trastuzumab at a 4 mg/kg intravenous loading dose followed by 2 mg/kg intravenously weekly for a total of 12 weeks, followed by a 6-week treatment-free interval, followed by lapatinib at 1,500 mg/d for 34 weeks total; or (4) trastuzumab at an 8 mg/kg intravenous loading dose followed by 6 mg/kg intravenously every 3 weeks concomitant with lapatinib at 1,000 mg/d for 52 weeks (later reduced to 750 mg/d when given concomitantly with chemotherapy).
- Design 2, concomitantly with a taxane following anthracycline: 80 mg/m2 weekly paclitaxel or 75 mg/m2 docetaxel every 3 weeks, concomitantly with the anti-HER2 agent.
- Design 2B: docetaxel at 75 mg/m2 for six cycles with carboplatin (AUC 6) concomitantly with the anti-HER2 agent; trastuzumab was given weekly, and lapatinib doses were reduced when combined with chemotherapy.
The majority (n = 4,613) received the anti-HER2 agents after completing chemotherapy. Patients with hormone receptor–positive cancers also received appropriate hormonal therapy.
Negative Findings
At a median follow-up of 4.5 years, dual targeting—either concurrently or sequentially—was associated with slight numerical reductions in disease recurrences, but the differences were not statistically significant, vs trastuzumab alone.
“The ALTTO trial did not meet its endpoints,” Dr. Piccart-Gebhart announced. Disease-free survival rates at 4 years were 86% with trastuzumab, 88% with concurrent HER2-directed treatment, and 87% in the sequential arm. Median overall survival rates were 94%, 95%, and 95%, respectively.
Hazard ratios ranged from 0.80 to 1.00, with none statistically significant. The analysis by hormone receptor status was similar. For the disease-free survival noninferiority analysis, the hazard ratio was 0.93 (P = .044 [P ≤ .025 required for statistical
significance]).
“The doubling in pathologic complete response observed with lapatinib plus trastuzumab in NeoALTTO did not translate into improved survival outcomes in ALTTO,” Dr. Piccart-Gebhart said.
Lapatinib More Toxic
“Lapatinib was also associated with significant increases in adverse events of special interesting—diarrhea, skin rash or erythema, and hepatobiliary problems,” Dr. Piccart-Gebhart said. The toxicity may account for the fact that only 60% to 78% of patients in the lapatinib-containing arms received at least 85% of the protocol-specified dose, she added.
Importantly, in ALTTO the rates of serious cardiotoxicity were very low—less than 1%—in spite of the fact that 97% of women received anthracyclines.
Correlative analyses of tissue samples is underway, and this may help to identify whether subsets of patients did benefit from the adjuvant combination. A protocol-specified updated efficacy analysis is planned in 2 years. ■
Disclosure: Dr. Piccart-Gebhart reported employment or leadership position with PharmaMar; a consulting or advisory role with Amgen, Astellas Pharma, AstraZeneca, Bayer, Invivis, Lilly, MSD, Novartis, Pfizer, Roche/Genentech, Sanofi, Symphogen, Synthon, and Verastem; and honoraria from Amgen, Astellas Pharma, AstraZeneca, Bayer, Invivis, Lilly, MSD, Novartis, Pfizer, Roche/Genentech, Sanofi, Symphogen, Synthon, and Verastem. Dr. Perez reported no potential conflicts of interest. For full disclosures of all the study authors, visit abstracts.asco.org.
References
1. Piccart-Gebhart MJ, Holmes AP, Baselga J, et al: First results from the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone, trastuzumab alone, their sequence, or their combination in the adjuvant treatment of HER2-positive early breast cancer. ASCO Annual Meeting. Abstract LBA4. Presented June 1, 2014.
2. Piccart-Gebhart M, Holmes AP, de Azambuja E, et al: The association between event-free survival and pathological complete response to neoadjuvant lapatinib, trastuzumab, or their combination in HER2-positive breast cancer. Survival follow-up analysis of the NeoALTTO study. 2013 San Antonio Breast Cancer Symposium. Abstract S1-01. Presented December 11, 2013.