Results of two pivotal breast cancer trials reported at the 2016 ASCO Annual Meeting confirmed the practice-changing findings that resulted from earlier findings.
PALOMA-2
The phase III PALOMA-2 trial confirmed results from the smaller, open-label phase II PALOMA-1 trial that led to the U.S. Food and Drug Administration (FDA) approval of the cyclin-dependent kinase 4/6 inhibitor palbociclib (Ibrance). The drug was approved in combination with letrozole for the first-line treatment of metastatic disease.1
“These data represent the longest front-line improvement in median progression-free survival seen to date in women with advanced estrogen receptor (ER)-positive breast cancer,” said Dennis Slamon, MD, Director of Clinical/Translational Research and Professor of Medicine at the University of California, Los Angeles, and Director of the Revlon/UCLA Women’s Cancer Research Program.

The outcomes of PALOMA-2 are everything you could possibly hope for, and they clearly justify the U.S. approval of this agent.— Nancy E. Davidson, MD
Tweet this quote
Nancy E. Davidson, MD, Director of the University of Pittsburgh Cancer Institute and a breast cancer expert, commented that the outcomes of PALOMA-2 are “everything you could possibly hope for,” and they “clearly justify the U.S. approval of this agent.… I’m sure that no one’s happier tonight than the FDA,” she said.
In 666 women with ER-positive, HER2-negative advanced breast cancer, first-line therapy with palbociclib combined with letrozole led to a greater than 10-month improvement in investigator-assessed median progression-free survival: 24.8 months vs 14.5 months for letrozole alone, translating into a highly significant 42% reduction in risk (hazard ratio [HR] = 0.58, P < .000001). Median follow-up for this analysis was approximately 23 months, as of data cutoff in February 2016.
“Blinded independent central review confirmed the progression-free survival advantage seen using investigator assessment,” he said. For this analysis, median progression-free survival was 30.5 vs 19.3 months (HR = 0.65, P = .0005). Objective response rates in the intent-to-treat population were 42% and 35%, respectively (odds ratio = 1.40, P = .0310).

These data represent the longest front-line improvement in median progression-free survival seen to date in women with advanced estrogen receptor–positive breast cancer.— Dennis Slamon, MD
Tweet this quote
In the previous PALOMA-1 trial, the improvement was also 10 months (HR = 0.49, P = .0004), he noted, adding that “consistent clinical benefit has been seen across PALOMA studies.” In PALOMA-2, “clinical benefit from palbociclib was also demonstrated across all prespecified subgroups,” he said.
The most common adverse event in the palbociclib arm was neutropenia (1.8%) and in the placebo arm was fatigue (0.9%). Serious adverse events were reported for less than 1% of patients in either treatment arm, except for febrile neutropenia, which occurred in 1.6% of the palbociclib arm vs none in the control arm, and pulmonary embolism, seen in 0.9% and 1.4%, respectively. Deaths due to adverse events were reported for 2.3% with palbociclib and 1.8% receiving placebo. One on-study death (pulmonary embolism/respiratory failure) in the placebo arm was considered to be treatment-related.
ACOSOG Z0011 Update
Armando Giuliano, MD, of Cedars-Sinai Medical Center, Los Angeles, reported an update of ACOSOG Z0011, a randomized trial of axillary node dissection in women with clinical T1/2N0M0 breast cancer with a positive sentinel node.2 In the initial report, the study found no significant difference in 5-year locoregional recurrence or survival between lumpectomy patients receiving axillary lymph node dissection and sentinel node dissection only.

Sentinel node dissection alone provides excellent 10-year locoregional control and survival, comparable to completion axillary lymph node dissection, for these selected patients, even with long-term follow-up.— Armando Giuliano, MD
Tweet this quote
“Sentinel node dissection alone provides excellent 10-year locoregional control and survival, comparable to completion axillary lymph node dissection, for these selected patients, even with long-term follow-up,” Dr. Giuliano concluded.
ACOSOG Z0011 randomly assigned 891 patients with positive sentinel nodes to completion (levels I and II) axillary lymph node dissection or sentinel node dissection only. After breast-conserving surgery and sentinel node dissection, patients who were node-positive (by hematoxylin-and-eosin stain) were randomized to axillary lymph node dissection or to no further surgery, both followed by whole-breast irradiation and systemic adjuvant treatment.
Of the 420 patients treated with axillary lymph node dissection, 106 (27.4%) had additional positive nodes removed beyond the sentinel node, and 10% of patients with sentinel node micrometastases had macrometastases removed with axillary lymph node dissection.
Patients were analyzed both with intent-to-treat analysis and by actual treatment received. There were no clinical differences between the intent-to-treat patients and treatment-received patients and no meaningful differences in the findings.
New Data in Breast Cancer
- The phase III PALOMA-2 trial evaluated palbociclib/letrozole vs letrozole alone in the first-line metastatic breast cancer setting and upheld results from PALOMA-1, which led to FDA approval of this combination.
- The doublet of palbociclib/letrozole improved median progression-free survival by approximately 10 months.
- The 10-year outcomes of ACOSOG Z0011 showed no benefit for axillary completion dissection over sentinel lymph node dissection alone in women with sentinel node-positive, T1-2N0M0 breast cancer.
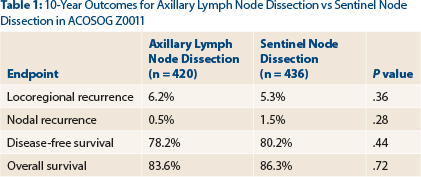
- Locoregional recurrence rates at 10 years were 6.2% with axillary lymph node dissection and 5.3% with sentinel node dissection (P = .36).
“The study has been criticized for its short initial follow-up of 6.3 years,” said Dr. Giuliano, who presented the 10-year outcomes by intent-to-treat analysis at the 2016 ASCO Annual Meeting. In the total population, 4.7% experienced a local recurrence and 1.0% had a regional recurrence by a median follow-up of 9.25 years. At 10 years, the two approaches still yielded comparable outcomes, as shown in Table 1.
Most locoregional recurrences were seen in the first 5 years. By year 5, there were two nodal recurrences with axillary lymph node dissection and four with sentinel node dissection. After 5 more years (by year 10), only one additional regional recurrence was observed, and this was in the sentinel node dissection–only arm.
Dr. Giuliano commented on the radiation protocol deviations. Of 605 radiation case reports reviewed, 11% of patients were found to have had no irradiation. Of 228 detailed records reviewed, 18.9% of patients received third-field irradiation (ie, encompassing more area than the protocol specified), but this was equally distributed between the two arms.
This unplanned analysis revealed increased local recurrence risk and reduced overall survival when radiation was omitted (P = .004). But radiation therapy was not associated with nodal recurrence (P = .80), and third-field irradiation was not associated with overall survival (P = .35).
“Hormone receptor status, Bloom-Richardson score, and tumor size—not the type of operation—were associated with locoregional recurrence,” he said. “And age, estrogen receptor status, tumor size, and adjuvant systemic therapy—not the operation—were associated with 10-year overall survival by multivariable analysis.”
“The routine use of axillary lymph node dissection should be abandoned,” Dr. Giuliano concluded. ■
Disclosure: Dr. Slamon reported financial relationships with BioMarin, Lilly, Pfizer, and Novartis. Drs. Davidson and Giuliano reported no potential conflicts of interest.
References

