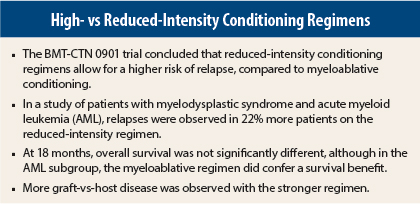
A randomized trial from the Bone and Marrow Transplant Clinical Trials Network was halted early after concluding that allogeneic stem cell transplantation after a reduced-intensity conditioning regimen resulted in higher relapse rates compared to myeloablative conditioning.
The phase III randomized study enrolled 54 patients with myelodysplastic syndrome (MDS) and 218 with acute myeloid leukemia (AML) who had < 5% marrow myeloblasts before transplant. The primary endpoint was overall survival at 18 months after randomization.
After showing a reduced risk of relapse with myeloablative conditioning though no difference in overall survival, the researchers concluded that “myeloablative conditioning remains the treatment choice over reduced-intensity conditioning for patients eligible to receive high-intensity–based regimens,” said Bart L. Scott, MD, of the Fred Hutchinson Cancer Research Center and the University of Washington in Seattle. Dr. Scott presented the findings at the late-breaking abstract session of the 2015 American Society of Hematology (ASH) Annual Meeting and Exposition.1
While reduced-intensity conditioning is associated with less toxicity and less treatment-related mortality, retrospective analyses have shown higher relapse rates and similar overall survival compared to myeloablative conditioning regimens—findings that the BMT-CTN 0901 trial sought to further clarify.
“Our hypothesis was that [reduced-intensity conditioning] would be associated with lower transplant-related mortality, and that would result in improved overall survival at 18 months,” Dr. Scott said.
BMT-CTN 0901 Details
The principal conditioning myeloablative conditioning regimen was 4-day busulfan (Busulfex, Myleran) with cyclophosphamide or fludarabine, whereas with reduced-intensity conditioning, it was 2-day busulfan.
The reduced-intensity conditioning regimens (n = 137) included these combinations:
- Fludarabine, 120–180 mg/m2, plus busulfan, ≤ 8 mg/kg orally or 6.4 mg/kg intravenously (Flu/Bu2; n = 110)
- Fludarabine, 120–180 mg/m2, plus melphalan, ≤ 150 mg/m2 (Flu/Mel; n = 27).
- The myeloablative conditioning regimens (n = 135) included:
- Fludarabine, 120–180 mg/m2, plus busulfan, 16 mg/kg orally or 12.8 mg/kg intravenously (Flu/Bu4; n = 87)
- Busulfan, 16 mg/kg orally or 12.8 mg/kg intravenously) plus cyclophosphamide, 120 mg/kg (Bu4/Cy; n = 40)
- Cyclophosphamide, 120 mg/kg, plus total-body irradiation, 1,200–1,420 cGy (Cy/TBI; n = 8).
The two arms were balanced in terms of age, gender, primary diagnosis, disease duration, World Health Organization classification, and risk according to cytogenetic and mutational profiles.
The planned enrollment was 356 patients. However, accrual was stopped early when the Data Safety and Monitoring Committee determined that myeloablative conditioning showed more benefit in terms of relapse reduction, Dr. Scott said.
Study Outcomes
At 18 months, overall survival was 77.4% with myeloablative conditioning and 67.7% with reduced-intensity conditioning—a difference of 9.7% that did not reach statistical significance (P = .07). By disease group, survival in patients with MDS was 81.5% with myeloablative conditioning and 85.2% with reduced-intensity conditioning—again, a nonsignificant difference (P = .71). However, for patients with AML, myeloablative conditioning yielded a statistically significant difference in survival: 76.8% at 18 months vs 63.0% for reduced-intensity conditioning (P = .027), Dr. Scott reported.
Relapse-free survival at 18 months, a secondary endpoint, was superior for myeloablative conditioning at 68.8%, vs 47.3% for reduced-intensity conditioning (P < .01). This held true for both disease subcategories.
Relapses occurred in 50% of AML patients receiving reduced-intensity conditioning, vs 16.5% receiving myeloablative conditioning. Among MDS patients, relapses occurred in 37% and 3.7%, respectively. Dr. Scott acknowledged, however, that the small number of patients, especially in the MDS subgroup, limited the study’s power to make conclusions about differences within subsets.
Transplant-related mortality at 18 months was significantly higher with myeloablative conditioning: 15.8% vs 4.4% (P = .02). The primary causes of death were graft-vs-host disease for myeloablative conditioning–treated patients and relapse for patients receiving reduced-intensity conditioning.
Reduced-intensity conditioning recipients had a longer time to neutrophil engraftment, a median of 19 days vs 15 days with myeloablative conditioning (P = .002) but a shorter time to platelet engraftment, 13 days vs 16 days (P = .065).
Acute graft-vs-host disease, grades 2 to 4, was more common among myeloablative conditioning recipients, who had a cumulative incidence of 44.7% at 100 days, compared to 31.6% in the reduced-intensity conditioning group (P = .024). Chronic graft-vs-host disease was also more common with myeloablative conditioning, 64% vs 47.6% at 18 months (P = .019).
“Novel, less toxic myeloablative conditioning regimens or more effective post-transplant maintenance regimens are needed to improve disease control in patients requiring [reduced-intensity conditioning],” Dr. Scott suggested.
Were Regimens Appropriately Categorized?
Tsiporah Shore, MD, of New York Presbyterian–Weill Cornell Medical Center, New York, felt that the categorization of the different regimens as myeloablative conditioning vs reduced-intensity conditioning was somewhat flawed, and this may have affected the findings.
“I am concerned that the range of regimens is too broad, when Flu/Mel is grouped together with the lower-level Flu/Bu2 regimen in the [reduced-intensity conditioning] arm, and the Flu/Bu4 regimen is put in the [myeloablative conditioning] arm,” she said during the discussion. She noted that studies recently found improved outcomes with Flu/Mel, compared to Flu/Bu.
Dr. Scott responded, “That’s an appropriate criticism. We can’t definitely state anything about Flu/Mel. The study was not powered to answer questions about the choice of the individual regimen.”
In an interview with The ASCO Post, Dr. Shore elaborated on her concerns. “The [myeloablative conditioning] arm contained very traditional, strong regimens as well as Flu/Bu4 (which delivered 4 days of busulfan). It’s somewhat myeloablative but perhaps not as strong as the other two. On the [reduced-intensity conditioning] arm we had Flu/Bu2 (which delivered 2 days of busulfan), and we had Flu/Mel. I consider Flu/Mel to be more like Flu/Bu4, but they listed it on the [reduced-intensity conditioning] side,” she noted.
“My center uses a lot of Flu/Mel, and we consider it to be pretty close to [myeloablative conditioning], with less toxicity. I think Flu/Mel got a bad rap by being in the [reduced-intensity conditioning] group, when they made a broad generalization that [reduced-intensity conditioning] is not strong enough to kill leukemia,” she said.
She added that multiple options needed to be offered, “to provide some level of comfort to clinicians,” who often have their preferences, “but I think it left the study open to some uncertainty.”
In response to Dr. Shore’s remarks, Dr. Scott told The ASCO Post: “Some centers may look at this study and state that there were very few patients who received melphalan, and maybe a melphalan-containing regimen would perform better. This is an appropriate question, but this study was not designed to answer that.… Previous publications from several centers have classified melphalan-based regimens as reduced intensity and not myeloablative. This was a multicenter study, and since many centers use melphalan as their reduced-intensity regimen, it was important to include this regimen in order to meet accrual goals.”
Dr. Shore was also disappointed that the study was terminated early, before all patients could be accrued. “If they had been able to look at overall survival as the endpoint, the number of relapses in the [reduced-intensity conditioning] arm may have been balanced out by more toxicity in the [myeloablative conditioning] arm, but the study didn’t have time to show this,” she added.
“The Data Safety and Monitoring Board had to do what they thought was appropriate, and I won’t question that, but it’s unfortunate that it left us not able to look at the endpoint we really wanted,” she said. ■
Disclosure: Drs. Scott and Shore reported no potential conflicts of interest.
Reference
1. Scott BL, Pasquini MC, Logan B, et al: Results of a phase III randomized, multicenter study of allogeneic stem cell transplantation after high versus reduced intensity conditioning in patients with myelodysplastic syndrome or acute myeloid leukemia: Blood and Marrow Transplant Clinical Trials Network 0901. 2015 ASH Annual Meeting. Abstract LBA-8. Presented December 8, 2015.