The formal discussant for the CALGB 40603 and GeparSixto studies at the San Antonio Breast Cancer Symposium was Angela DeMichele, MD, Professor of Medicine and Miller Chair in Breast Cancer Excellence at the Perelman School of Medicine, University of Pennyslvania.
“The key questions raised by these trials,” she said, “are whether they provide sufficient data to warrant routine use of carboplatin in early triple-negative breast cancer and whether [pathologic complete response] is a valid surrogate for outcomes.”
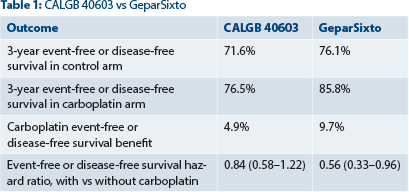
Dr. DeMichele noted that GeparSixto yielded better overall prognosis and a larger, incremental carboplatin effect (Table 1) and suggested this may be due to a more effective dosing schedule. The chemotherapy backbone and carboplatin dose and schedule may be critical to its optimal efficacy, she said.
Whether the data warrant routine use of carboplatin in triple-negative breast cancer remains “an individualized decision,” she added. “The hazard ratios suggest benefit, but currently there are not enough data to be conclusive.”
Regarding the use of pathologic complete response as a surrogate for outcomes in early triple-negative disease, she noted, “The hazard ratios are in the right direction despite the small sample sizes and variability in event-free survival estimates.” She gave this question “a qualified yes.”
The data at least contribute to the development of models to better understand this relationship, “but to draw definitive conclusions about event-free survival, we need to power neoadjuvant studies for these endpoints, Dr. DeMichele concluded.
Meanwhile, Dr. DeMichele advised that carboplatin can be considered outside of clinical trials in patients who need rapid control of locoregional disease (to increase operability and reduce morbidity) or have the highest risk of relapse (stage III, very young). Benefit to BRCA mutation carriers is still under investigation, she added, and she emphasized the need to carefully select patients for carboplatin, since it may contribute to short-term and possibly long-term toxicity.
‘Incredibly Important Question’
Also weighing in was Melinda L. Telli, MD, Assistant Professor of Medicine at Stanford University School of Medicine, Palo Alto, who has spearheaded research in the area of triple-negative disease. In an interview with The ASCO Post, Dr. Telli agreed with many of Dr. DeMichele’s points, including the suggestion that the improvement seen in GeparSixto may be due to its more intensive chemotherapy regimen.
“The German study looked at a somewhat unconventional, very dose-intensive regimen of 18 weeks of a taxane/anthracycline with bevacizumab [Avastin], with and without carboplatin. It showed an elevation in [pathologic complete response] rate and a 3-year disease-free survival difference of about 10% that appeared to clearly favor the carboplatin-treated patients,” she said.
The CALGB study, however, used an anthracycline/cyclophosphamide/taxane backbone, with or without carboplatin, which is more familiar to U.S. clinicians. This resulted in increased pathologic complete response rates but no improvement in long-term outcomes.
Dr. Telli said it is possible that the “carboplatin made up ground” for the lack of an alkylating agent in GeparSixto. “Cyclophosphamide wasn’t included, so potentially the platinum filled that role. The point was also raised that the intensive weekly regimen given for 18 cycles led to higher cumulative doses of the different drugs,” she noted.
“At the end of the day, we need a bigger study,” she said. To this end, carboplatin is being evaluated in adjuvant, postadjuvant, and neoadjuvant trials of triple-negative breast cancer, including the neoadjuvant Brightness trial of standard taxane followed by doxorubicin/cyclophosphamide chemotherapy with and without carboplatin and with and without veliparib.
“It’s a big study that is better powered for long-term outcomes. Maybe that study will give us better answers,” Dr. Telli said.
The value of adding carboplatin is “an incredibly important question,” she concluded. “Certainly if there’s a drug that is inexpensive and that can improve disease-free survival by 10% in this high-risk group of patients, that would be great. But I’m not going to change my practice yet.” ■
Disclosure: Drs. DeMichele and Telli reported no potential conflicts of interest.