
Thierry André, MD
IN THE FIRST REPORT of the full cohort of CheckMate-142, nivolumab (Opdivo) plus ipilimumab (Yervoy) led to a 1-year overall survival rate of 85% in previously treated patients with metastatic colorectal cancer who have DNA mismatch repair–deficient (dMMR) or microsatellite instability–high (MSI-H) tumors. Median overall survival and progression-free survival have not yet been reached at a median follow-up of 13.4 months, reported Thierry André, MD, of the Saint-Antoine Hospital in France, at the 2018 Gastrointestinal Cancers Symposium.1
Durable clinical benefit was also achieved with single-agent nivolumab, based on longer follow-up of that cohort. “Progression-free and overall survival demonstrated continued stability. Complete response rates increased, and the median duration of response and overall survival were not yet reached,” Michael J. Overman, MD, of The University of Texas MD Anderson Cancer Center, Houston, reported at the Symposium.2

Michael J. Overman, MD
CheckMate-142 Details
CHECKMATE-142 is a nonrandomized phase II study designed to determine, within the dMMR/MSI-H population, whether nivolumab alone or in combination with another checkpoint inhibitor has activity against colorectal cancer that has metastasized or recurred following at least one prior treatment. The study enrolled 74 patients, who received nivolumab as a single agent (3 mg/kg every 2 weeks) and 119 patients who received nivolumab (3 mg/kg) plus ipilimumab (1 mg/kg) every 3 weeks for four doses, followed by nivolumab (3 mg/kg) every 2 weeks.
CheckMate-142 is the largest single study of combination immune checkpoint inhibitors in patients with dMMR/MSI-H metastatic colorectal cancer. Data for the combination and for the single-agent arm were presented at the meeting, but the two arms were not directly compared.
Nivolumab and ipilimumab act synergistically to promote T-cell antitumor activity, providing the rationale for trying to improve upon nivolumab’s single-agent activity. Based on initial data from CheckMate-142, the U.S. Food and Drug Administration granted accelerated approval to nivolumab for patients with dMMR/MSI-H colorectal cancer who have disease progression after treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.
1-Year Survival of 85%
DR. ANDRÉ PRESENTED the findings for the combination treatment arm, after a median follow-up of 13.4 months. The objective response rate was 55%, with 3.4% being complete responses, and the disease control rate was 80%. Some degree of tumor reduction was achieved by 78% of patients.
Responses were observed regardless of tumor programmed cell death ligand 1 (PD-L1) expression, BRAF or KRAS mutation status, or a clinical history of Lynch syndrome. The median time to response was 2.8 months (range, 1–14 months), and the median duration of response has not yet been reached. Some responses occurred “very late,” noted Dr. André.
Among patients who responded to the combination, 94% were still responding at the time of data cutoff, and at the time of this report, 63% of the nivolumab/ipilimumab cohort remained on treatment. These results are particularly striking considering 76% of these patients had received two or more prior lines of therapy, he added.
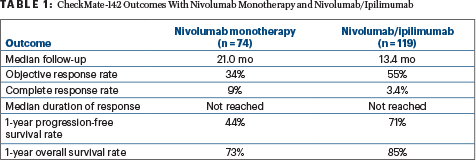
Combined treatment with nivolumab/ipilimumab also provided impressive benefits in progression-free and overall survival, with rates at 1 year of 71% and 85%, respectively. “The progression-free survival curve is a plateau,” Dr. André observed, “which is very unusual to have in advanced disease…. Relative to monotherapy, with similar follow-up, combination therapy provided improved progression-free and overall survival.”
By two formal instruments, “Statistically significant and clinically meaningful improvements in quality-of-life measures were seen and were maintained for extended periods while patients remained on treatment,” Dr. André revealed. “This is the first time I have had a very large number of patients going back to work with this very advanced disease.”
No new safety signals emerged with the addition of ipilimumab to nivolumab, and the rates of all-grade adverse events were similar to those seen with the single agent, 73% vs 70%, respectively. A modest increase in the rate of grade 3/4 treatment-related adverse was seen, 32% vs 20%, respectively. Treatment-related adverse events leading to drug discontinuation occurred in 13% and 7%, respectively. The most common toxicities with the combination were diarrhea (22%), fatigue (18%), pruritus (17%), and pyrexia (15%).
Longer Follow-up of Nivolumab Monotherapy
WHILE COMBINATION THERAPY provided numerically higher rates of partial and complete responses and disease control, nivolumab monotherapy also showed activity in CheckMate-142. With 21 months of follow-up, responses have deepened, Dr. Overman reported. His report was an update on the 74 patients in the monotherapy arm, including 53 who received 3 or more prior chemotherapies (including a fluoropyrimidine, oxaliplatin, and irinotecan) and 21 who had not received all three drugs.
In the previously reported initial analysis, performed after 13 months of median follow-up, the objective response rate per blinded independent central review was 32%, with 73% of patients alive at 1 year.3 Now after 21 months, the response rate was 34%, with 9% being complete responses, and the disease control rate was 62%. By subgroup, response rates were 26% for patients previously treated with three drugs and 52% for patients who had received fewer treatments; disease control rates were 55% and 81%, respectively.
NIVOLUMAB IN dMMR/MSI-H TUMORS
- CheckMate-142 evaluated nivolumab as monotherapy or in combination with ipilimumab in previously treated patients with metastatic colorectal cancer who have dMMR/MSI-H tumors.
- After a median follow-up of 21 months, single-agent nivolumab produced a 34% response rate. Responses were durable, lasting at least 1 year in 64% of patients. At 1 year, 44% were progression-free and 73% were alive.
- After a median follow-up of 13.4 months, response rates with nivolumab/ ipilimumab were 55%. At 1 year, 71% were progression-free and 85% were alive.
- The study is not formally comparing the two arms, but outcomes are numerically higher with the combination.
The median duration of response was still not reached in the overall cohort or in the two subgroups (by prior treatment). Among responders, 80% had ongoing responses at the time of data cutoff, and 64% had responses lasting at least 1 year.
Dr. Overman emphasized the complete response rate deepened with longer follow-up. Whereas only 3% of patients had attained a complete response to nivolumab monotherapy by 13 months, this number rose to 9%, after a median of 21 months of follow-up. Similar trends in complete response were seen in both subgroups.
The depth of response was clearly related to survival. “None of the responders (complete and partial) have died yet, and for patients with stable disease, median survival has not been reached,” he noted. “We see extremely good long-term, durable outcomes.”
Median progression-free survival for the overall cohort was 6.6 months, but Dr. Overman maintained this number “does not well represent the benefit from this therapy.” Instead, he pointed to the “flattening of the curve with further follow-up,” noting the progression-free survival rate was exactly the same at 18 months as at 12 months: 44%. Similarly, median overall survival has not been reached and is also similar at 12 months (73%) and 18 months (67%), demonstrating the durability of response. A small difference in 18-month survival was found for patients who had received fluoropyrimidine, oxaliplatin, and irinotecan (66%) vs those who had not (70%).
No new safety signals emerged with long-term follow-up. Twenty percent of patients experienced grade 3/4 treatment-related adverse events, the most common being increased lipase level (8%). ■
DISCLOSURE: Dr. André is a consultant for and is on the advisory board of Bristol-Myers Squibb. Dr. Overman has served as a consultant or advisor to Bristol-Myers Squibb, Merrimack Pharmaceuticals, and Roche/Genentech.
REFERENCES
1. André T, Lonardi S, Wong M, et al: Nivolumab + ipilimumab combination in patients with DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer: First report of the full cohort from CheckMate-142. 2018 Gastrointestinal Cancers Symposium. Abstract 553. Presented January 20, 2018.
2. Overman MJ, Bergamo F, McDermott RS, et al: Nivolumab in patients with DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer: Long-term survival according to prior lines of treatment from CheckMate-142. 2018 Gastrointestinal Cancers Symposium. Abstract 554. Presented January 20, 2018.
3. Overman MJ, McDermott R, Leach JL, et al: Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142). Lancet Oncol 18:1182-1191, 2017.


