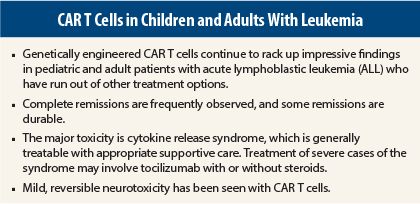
As more experience is gained with the use of genetically engineered chimeric antigen receptor (CAR) T cells in patients with leukemia, the data continue to be highly encouraging. Three different groups using slightly different modifications of CAR T cells reported positive experiences in treating acute lymphocytic leukemia (ALL) at the 56th American Society of Hematology (ASH) Annual Meeting and Exposition.1-3
Pediatric Relapsed/Refractory Acute Lymphocytic Leukemia
To date, 130 patients have received CAR T cells (CTL019) at the University of Pennsylvania, including those with ALL, chronic lymphocytic leukemia (CLL), and non-Hodgkin lymphoma (NHL).
Stephan Grupp, MD, of Children’s Hospital of Philadelphia Perelman School of Medicine at the University of Pennsylvania, Philadelphia, reported his experience with CTL019 in 39 pediatric patients with ALL.1 Thirty-six of these patients (92%) have achieved complete remission, and responses are durable. At a median follow-up of 6 months, some patients had a remission of 1 year or more. The 6-month event-free survival was 70%. The overall survival was 75%.
“We are seeing pediatric patients who have not responded to other therapies achieve extended complete remissions as a result of treatment with CTL019,” Dr. Grupp said.
Another important finding is that after the genetically engineered T cells are reinfused into the patient, they continue to persist in vivo for an “extraordinary” length of time, up to 31 months in ongoing responders. Expansion, proliferation, and persistence are accompanied by B-cell aplasia, a pharmacodynamic marker of CTL019 persistence and function, considered an adverse event and managed with intravenous immunoglobulin replacement therapy.
Cytokine Release Syndrome
The major toxicity of concern is cytokine release syndrome, which was seen in all responding patients at peak T-cell expansion. Symptoms of cytokine release syndrome include varying degrees of flu-like symptoms, with a high fever, nausea, muscle pain, and in some cases low blood pressure and respiratory distress.
Treatment of severe cytokine release syndrome was required in 33% of the pediatric patients. Fortunately, this syndrome resolves within 1 to 2 days with the use of tocilizumab (Actemra), an interleukin 6–receptor antagonist, Dr. Grupp told listeners.
The severity of cytokine release syndrome appears to be related to disease burden at the time of infusion; patients with a higher disease burden are much more likely to develop severe cytokine release syndrome, Dr. Grupp said.
Other toxicities include macrophage activation syndrome and neurotoxicity in a small number of patients after the occurrence of cytokine release syndrome.
Manufacturing CAR T cells is labor-intensive, involving extracting the patient’s own T cells and genetically engineering them to target the CD19 surface antigen on tumor cells. The T cells are then reinfused into the patient, unleashing cytotoxicity against tumor cells in an antigen-dependent manner. Dr. Grupp said that ongoing research is being devoted to expediting the manufacturing process so that CAR T cells can become more widely available.
Different Version of CAR T Cells
Results of a phase I trial using a slightly different version of CAR T cells achieved a 70% complete response rate in 20 patients with ALL (in our intent-to-treat analysis, including 1 patient with lymphoma, the overall complete response rate was 67%), and the vast majority were minimal residual disease–negative,2 reported Daniel “Trey” Lee, MD, of the National Cancer Institute, Bethesda, Maryland.
The genetically engineered CD19 T cells used in this trial are developed with a retroviral platform and a different costimulatory domain instead of a lentivirus platform, as is used by the group at University of Pennsylvania and Children’s Hospital of Pennsylvania.
The intent-to-treat study was designed to assess the safety and feasibility of CAR T cells in 21 patients, aged 1 to 30 years. Six patents had primary refractory disease and never achieved minimal residual disease–negative status on previous treatments. All were heavily pretreated, and eight had prior stem cell transplants.
Regardless of prior transplant, peripheral blood mononuclear cells were collected and CAR T cells manufactured and reinfused into patients 11 days after collection. Patients were lymphodepleted with fludarabine and cyclophosphamide prior to cell infusion.
Among minimal residual disease–negative patients, 79% were leukemia-free at at a median follow-up of 10 months. For all patients, overall survival was 52% at a median follow-up of 10 months.
As with the University of Pennsylvania experience, Dr. Lee reported a significant frequency of cytokine release syndrome with in vivo proliferation of CAR T cells. These episodes rapidly resolved after treatment with appropriate supportive care or, for more severe cases, tocilizumab and corticosteroids. Other adverse events included mild, reversible neurotoxicity, Dr. Lee said. “Our CAR appears to be less toxic, since our severe cytokine release syndrome rate was 14% in contrast to the 33% seen by Dr. Grupp’s group,” he noted.
CAR T cells were found in the cerebrospinal fluid of patients, and the phase I trial has expanded eligibility to include patients with isolated and/or high levels of central nervous system leukemia.
Adult Relapsed/Refractory ALL
Durable responses have been observed in adult patients with relapsed/refractory B-cell ALL treated with CAR T cells at Memorial Sloan Kettering Cancer Center in New York.
“The prognosis of adult ALL remains poor, with a 5-year overall survival of 7% in patients in first relapse and 3% in those over age 50. Conventional chemotherapy will achieve a response rate of 20% to 37% for patients in first relapse, but the rate is lower in older patients and those with poor-risk cytogenetics. We need to do a better job in adults with relapsed ALL,” said lead author Jae Park, MD, of Memorial Sloan Kettering Cancer Center.
Dr. Park and colleagues used CD19-28z–directed CAR T cells using a retroviral vector. He reported results of a phase I trial in 33 adult patients older than age 18 with relapsed/refractory B-cell ALL and no central nervous system involvement.3
About 75% were male, the median age was 55 years (range, 23–74 years), 32% were older than age 60, and 46% had minimal disease (< 5% blasts). Two-thirds of patients had two prior lines of therapy, 18% had three prior lines, and the remaining patients had more than three prior therapies.
Of 27 patients evaluable for response, 24 had a complete response (89%), and 21 were minimal residual disease–negative (88%). The median time to complete remission was 22.5 days.
Complete remission was maintained regardless of prior disease-risk characteristics. At a median follow-up of 6 months (range, 1–38 months), 12 patients remained disease-free (7 without subsequent stem cell transplant) for up to 1 year or more. Ten patients went on to stem cell transplant, and nine relapsed during follow-up.
The median overall survival of all patients is 8.5 months; at 6 months, 57% were alive. In patients who went on to allogeneic transplant, the median overall survival is 10.8 months; 6-month survival is 68%.
In this study, experience regarding cytokine release syndrome and neurotoxicity was similar to that in the other two reports. ■
Disclosure: Dr. Grupp is a consultant and has received research funding from Novartis. Dr. Lee reported no potential conflicts of interest. Dr. Park has received research funding from Juno Therapeutics.
References
1. Grupp SA, et al: 2014 ASH Annual Meeting. Abstract 380. Presented December 8, 2014.
2. Lee DW, et al: 2014 ASH Annual Meeting. Abstract 381. Presented December 8, 2014.
3. Park JH, et al: 2014 ASH Annual Meeting. Abstract 382. Presented December 8, 2014.