An investigational class of agents in multiple myeloma, the anti-CD38 monoclonal antibodies, could be the next blockbusters in this malignancy, myeloma experts predicted at the 56th American Society of Hematology (ASH) Annual Meeting and Exposition.
Anti-CD38 antibodies target multiple myeloma cells by binding to the CD38 antigen expressed on the cell surface and then signaling the patient’s immune system to attack the tumor. Although the data reported at the ASH meeting came from only phase Ib studies, the findings were considered impressive.
Despite the success of the proteasome inhibitors and immunomodulating drugs, there are still unmet needs that might be filled by new classes of agents, said Thomas Martin, MD, of the University of California, San Francisco. “These anti-CD38 monoclonal antibodies, I believe, will be blockbuster drugs and an important component of multiple myeloma treatment,” he predicted. “Clinicians should definitely be referring patients to these trials. We finished ours in record time. Doctors were on the telephone line, trying to get a spot on our trial.”
Press briefing moderator Brad Kahl, MD, Associate Professor of Medicine at the University of Wisconsin in Madison, commented, “Obviously, it’s very, very early with these drugs, and it’s too early to plant the victory flag in the ground, but having said that, the early data are quite promising and justify bringing these drugs into the front-line setting.”
SAR650948 in Heavily Pretreated Patients
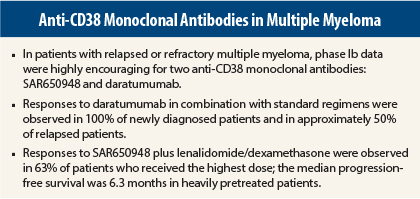
Dr. Martin presented the results from a dose-escalation study of SAR650948,1 which was given in combination with a standard regimen of lenalidomide (Revlimid)/dexamethasone in 31 patients with relapsed/refractory disease. There was no upper limit on the number of prior lines of therapy.
“These were very heavily pretreated patients,” he noted. “More than 90% had prior lenalidomide, 90% had prior bortezomib (Velcade), about 30% had prior pomalidomide (Pomalyst), and 50% had prior carfilzomib (Kyprolis) exposure. Many patients were double-refractory.”
The combination produced responses in 58% of patients overall, rising to 63% in patients receiving the highest dose, 10 mg/kg every 2 weeks. The clinical benefit rate was 65% overall and 67% with the maximum dose.
Responses were achieved in 50% of patients who had relapsed on or were refractory to previous treatment with immunomodulating drugs, in 44% who had relapsed on or were refractory to bortezomib, 40% who had relapsed on or were refractory to carfilzomib, and 33% who had relapsed on or were refractory to pomalidomide.
Referring to the robust reduction in the M protein, a marker of disease, Dr. Martin observed the waterfall plot to be “dramatic.” Only 4 of the 31 patients did not demonstrate at least a 25% reduction in paraprotein, and the majority of patients achieved at least a 50% reduction.
The median progression-free survival was 6.2 months, but it had not been reached in patients who had received only one or two prior lines of therapy. The median duration of response was 9 months.
“In my mind, SAR650984 in combination with lenalidomide and dexamethasone produced fairly dramatic responses,” he said. “In the relapsed/refractory setting, you must look at prior treatments, and this was a very refractory group.”
The addition of SAR650984 did not increase toxicity. The pharmacokinetics of SAR650984 and lenalidomide appear to be independent, he noted.
The most common adverse events were mild fatigue, nausea, diarrhea, neutropenia, and upper respiratory infection. Two patients discontinued treatment due to infusion-related reactions, but most such reactions resolved after one cycle.
“These are the next blockbusters, drugs that will show benefit in myeloma, and in the next 5 years, I think we will see them moved from the refractory setting, even to front line eventually, and that will be great,” Dr. Martin commented at a press briefing.
Daratumumab Tested in Four Combinations
Philippe Moreau, MD, of the University Hospital in Nantes, France, reported preliminary data for a second anti-CD38 antibody, daratumumab. Daratumumab, which had shown single-agent activity, was combined with one of four standard regimens in the treatment of 18 newly diagnosed patients and 6 relapsed/refractory patients in the MMY1001 study.2 Regimens included bortezomib/dexamethasone, bortezomib/thalidomide (Thalomid)/dexamethasone, bortezomib/melphalan/prednisone, and, for previously treated patients, pomalidomide/dexamethasone.
Fully 100% of newly diagnosed patients responded to daratumumab (16 mg/kg) plus a standard regimen. The response rate was 50% in the relapsed group, Dr. Moreau noted.
After a median treatment duration of 44 days, no additional toxicity was observed with the addition of daratumumab to these regimens. The most serious adverse events were related to the backbone therapy. Four patients had infusion reactions, but they did not interrupt treatment. All other adverse events were consistent with those previously reported with the standard regimens.
Torben Plesner, MD, of Vejle Hospital in Denmark, also reported findings on daratumumab from an international study of 45 relapsed/refractory patients who received daratumumab with lenalidomide plus dexamethasone.3
“Daratumumab plus lenalidomide leads to enhanced killing of myeloma cells in vitro and is hypothesized to lead to synergistically higher efficacy in the clinical setting,” Dr. Plesner indicated.
Patients had received a median of two prior lines of therapy, and three of them were refractory to lenalidomide.
The overall response rate was 100% in the dose-escalation phase with median follow-up duration of 13 months and 87% in the expansion phase of the study with median follow-up duration of 6 months. In patients who were treated for at least 6 months, 75% achieved a very good partial response or better. The majority of patients had at least a 50% reduction in M protein.
“The data from part 1 are mature and show an impressive rate of complete response (31%),” Dr. Plesner reported. “And the early results from part 2 are consistent with part 1. Our median follow-up is still less than 6 months and only 2.7 months for the last patient dosed in this analysis.”
Of 45 patients, 19 had infusion reactions, but most (86%) occurred during the first infusion only, and all but one patient recovered and were able to continue with subsequent infusions.
Phase III clinical development of daratumumab in combination with lenalidomide/dexamethasone is ongoing in both the relapsed/refractory and front-line settings.
Paul Richardson, MD, Clinical Program Director and Director of Clinical Research at the Jerome Lipper Multiple Myeloma Center at Dana-Farber Cancer Institute, Boston, who moderated the oral session, called the data “remarkable.” He said, “monoclonal antibodies have activity in high-risk disease and represent truly novel mechanisms of action” and suggested that the combination of monoclonal antibodies and pomalidomide will produce excellent outcomes in the future. ■
Disclosure: Dr. Martin has received research funding from Sanofi and is on the speakers bureau for Novartis. Dr. Moreau has received honoraria from Janssen and is a member of Janssen’s Board of Directors or advisory committees. Dr. Plesner is a consultant with Genmab and a member of Janssen’s and Celgene’s Board of Directors or advisory committees. Dr. Richardson reported no potential conflicts of interest.
References
1. Martin TG III, Baz R, Benson DM, et al: A phase 1b dose escalation trial of SAR650984 (anti-CD-38 mAb) in combination with lenalidomide and dexamethasone in relapsed/refractory multiple myeloma. 2014 ASH Annual Meeting. Abstract 83. Presented December 7, 2014.
2. Moreau P, Mateos M-V, Bladé J, et al: An open-label, multicenter, phase 1b study of daratumumab in combination with backbone regimens in patients with multiple myeloma. 2014 ASH Annual Meeting. Abstract 176. Presented December 7, 2014.
3. Plesner T, Arkenau H-T, Lokhorst HM, et al: Safety and efficacy of daratumumab with lenalidomide and dexamethasone in relapsed or relapsed, refractory multiple myeloma. 2014 ASH Annual Meeting. Abstract 84. Presented December 7, 2014.