Question 1: Based on the rationale for the current “standard of care” for primary diffuse large B-cell lymphoma of the CNS, what is the optimal induction therapy?
Correct Answer: C. A high-dose methotrexate–based regimen.
Expert Perspective
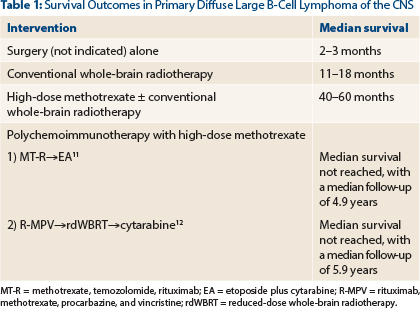
Untreated primary diffuse large B-cell lymphoma of the CNS is associated with dismal outcomes, with a median survival of 2–3 months.1-3 Corticosteroids can provide both rapid clinical and radiographic improvement when used alone, but they rarely lead to a sustained response. Steroids are included as a component of a number of polychemotherapy regimens for primary diffuse large B-cell lymphoma of the CNS.
Radiation alone is associated with high response rates, but 66% of patients recur within the tumor bed; this results in disappointing progression-free survival rates and a median overall survival in the range of 11–18 months, leaving few (1%–2%) long-term survivors.4,5 However, whole-brain radiotherapy alone remains a viable option in patients deemed too weak to tolerate high-dose methotrexate.
In a multivariate analysis, high-dose methotrexate has been shown to be the only treatment-related factor independently associated with improvement in survival (P = .01)6; however; single-agent high-dose methotrexate alone is not a sufficient induction approach in otherwise “fit” patients with diffuse large B-cell lymphoma of the CNS.7 Therefore, efforts have been focused on further increasing treatment efficacy by adding other active agents or strategies during induction (with high-dose methotrexate) and consolidation (after high-dose methotrexate) phases of therapy.
The earliest use of a methotrexate-based regimen was intravenous methotrexate at doses of 200 mg/m2 and 1 g/m2 in conjunction with intrathecal methotrexate followed by whole-brain radiotherapy and high-dose cytarabine.8 This approach was further evaluated in a larger group of patients (n = 102) in a multicenter trial (RTOG 9310), where high-dose methotrexate (2.5 g/m2) in conjunction with intrathecal methotrexate, vincristine, and oral procarbazine were followed by whole-brain radiotherapy and cytarabine.9
The overall survival and progression-free survival were 36.9 and 24.0 months, respectively. A total of 58% of patients experienced complete response, with an additional 36% of patients experiencing partial responses, prior to delivery of whole-brain radiotherapy. Older age and intervention with whole-brain radiotherapy, even at a lower dose of 36 Gy (decreased from the original 45 Gy dose in this trial), did not prevent treatment-related deleterious neurotoxicity. Approximately 24% of patients treated with combined chemoradiotherapy for diffuse large B-cell lymphoma of the CNS developed serious neurotoxicity, which increased over time.10 Thus, optimal treatment for patients with diffuse large B-cell lymphoma is poorly defined, due to a lack of randomized phase III trials.
Furthermore, a direct comparison between the specific components of high-dose methotrexate–based regimens studied in different trials is not possible. For example, many of the regimens included whole-brain radiotherapy consolidation or utilized intrathecal chemotherapy during induction. However, despite these limitations in data, a number of recent phase II clinical trials have documented continued improved outcomes with high-dose methotrexate–based regimen(s), establishing high-dose methotrexate as a “standard part of induction therapy.”
Our patient started induction therapy as outlined in the CALGB 50202 (Alliance 50202) trial. This induction regimen (which included high-dose methotrexate, leucovorin, rituximab, and temozolomide) has proved to be effective and well tolerated in adults up to age 74.11 Following induction therapy, the disease is re-assessed, and in this particular protocol, consolidation therapy with cytarabine and etoposide is delivered to patients who achieve complete response or unconfirmed complete response.
The CALGB 50201 (Alliance 50202) protocol successfully eliminated the risk of radiation-induced neurotoxicity by omitting consolidative whole-brain radiotherapy without jeopardizing outcomes (Table 1). Whole-brain radiotherapy still may have an important therapeutic position, albeit at a lower dose than 30 Gy when combined with polychemoimmunotherapy.
For example, a multicenter phase II study administered rituximab, methotrexate, procarbazine, and vincristine followed by consolidation with reduced-dose whole-brain radiotherapy (23.4 Gy) and cytarabine. This regimen resulted in encouraging outcomes with minimal neurotoxicity.12 However, on this protocol, older (> 60 years) adults had inferior progression-free survival compared to younger adults.
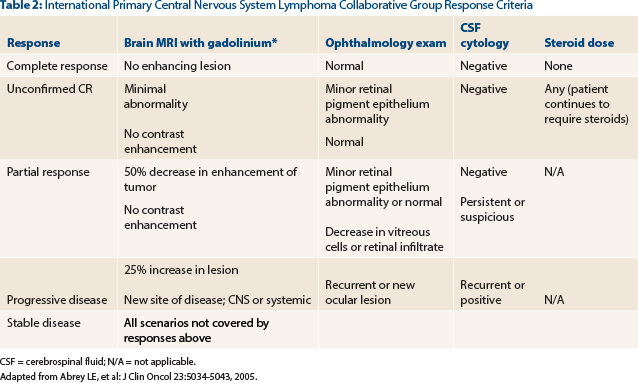
Question 2: This patient would be classified as having which of the following International Primary Central Nervous System Lymphoma Collaborative Group response criteria?
Correct Answer: B. Unconfirmed complete response.
Expert Perspective
The patient has demonstrated a near resolution of all radiographic abnormalities. The response noted on his scan is greater than the ≥ 50% reduction in the cross-sectional area of enhancing tumor necessary for a partial response. A complete response would require a complete resolution of the enhancement in addition to negative cerebrospinal fluid cytology (if previously positive) and negative ophthalmologic slit lamp examination—all while the patient is not on steroids for at least 2 weeks. Due to the small amount of residual enhancement likely representative of gliosis at the biopsy site, our patient is categorized as having an unconfirmed complete response13,14 (Table 2).
Question 3: What is the optimal consolidation therapy?
Correct Answer: C. Evaluation in a National Cancer Institute–sponsored cooperative group phase II trial.
Expert Perspective
There remain many unanswered questions in the management of primary diffuse large B-cell lymphoma of the CNS. This question highlights controversies related to consolidation therapy in patients with this type of lymphoma. At present, the optimal consolidation therapy for patients with primary diffuse large B-cell lymphoma of the CNS is not clear. As previously stated, high-dose methotrexate–based regimens have utilized both chemotherapy and whole-brain radiotherapy as part of consolidation therapy, with favorable outcomes (Table 1). However, in older adults (particularly those ≥ age 60), there are substantial concerns of deleterious neurotoxicity with whole-brain radiotherapy.10 In addition, consolidation with polychemotherapy (ie, etoposide plus cytarabine) in older adults did not adversely affect outcomes.11
For these multiple reasons, we utilized the CALGB 50201 (Alliance 50202) protocol for our patient outside of a clinical trial. Other viable options exist as well for this patient population. There are two ongoing phase II National Cancer Institute–sponsored cooperative group studies evaluating the role of different treatment modalities for consolidation after predefined induction chemoimmunotherapies.
1) CALGB 51101 (NCT01511562): MT-R followed by consolidative EA vs high-dose chemotherapy using carmustine and thiotepa with autologous cell rescue for patient between the ages of 18 and 75.
2) RTOG 1114 (NCT01399372): R-MPV followed by cytarabine consolidation vs the same regimen with the addition of rdWBRT (of 23.4 Gy) in 13 fractions prior to cytarabine for patients older than age 18.
Our patient was offered the choice of the CALGB 51101 trial, but he elected to be treated off trial on the CALGB 50201 (Alliance 50202) protocol. After completing consolidation therapy with etoposide plus cytarabine, he remains in remission and is scheduled for a clinic visit soon. ■
Disclosure: Drs. Abutalib and Lukas reported no potential conflicts of interest.
References
1. Abrey LE, Ben-Porat L, Panageas KS, et al: Primary central nervous system lymphoma. J Clin Oncol 24:5711-5715, 2006.
2. Ferreri AJ, Blay JY, Reni M, et al: Prognostic scoring system for primary CNS lymphomas. J Clin Oncol 21:266-272, 2003.
3. Ney DE, Deangelis LM: Management of CNS lymphoma, in Armitage JO, Mauch PM, Harris NL, et al (eds): Non-Hodgkin Lymphomas, 2nd ed, ch 36, pp 527-539. Philadelphia, Lippincott Williams & Wilkins, 2014.
4. Nelson DF, Martz KL, Bonner H, et al: Non-Hodgkin’s lymphoma of the brain: Can high dose, large volume radiation therapy improve survival? Int J Radiat Oncol Biol Phys 23:9-17, 1992.
5. Shibamoto Y, Ogino H, Hasegawa M, et al: Results of radiation monotherapy for primary central nervous system lymphoma in the 1990s. Int J Radiat Oncol Biol Phys 62:809-813, 2005.
6. Blay JY, Conroy T, Chevreau C, et al: High-dose methotrexate for the treatment of primary cerebral lymphomas. J Clin Oncol 16:864-871, 1998.
7. Batchelor T, Carson K, O’Neill A, et al: Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy. J Clin Oncol 21:1044-1049, 2003.
8. DeAngelis LM, Yahalom J, Heinemann MH, et al: Primary CNS lymphoma. Neurology 40:80-86, 1990.
9. DeAngelis LM, Seiferheld W, Schold SC, et al: Combination chemotherapy and radiotherapy for primary central nervous system lymphoma. J Clin Oncol 20:4643-4648, 2002.
10. Omuro AM, Ben-Porat LS, Panageas KS, et al: Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol 62:1595-1600, 2005.
11. Rubenstein JL, Hsi ED, Johnson JL, et al: Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma. J Clin Oncol 31:3061-3068, 2013.
12. Morris PG, Correa DD, Yahalom J, et al: Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma. J Clin Oncol 31:3971-3979, 2013.
13. Küker W, Nagele T, Thiel E, et al: Primary central nervous system lymphomas. Neurology 65:1129-1131, 2005.
14. Abrey LE, Batchelor TT, Ferreri AJ, et al: Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 23:5034-5043, 2005.