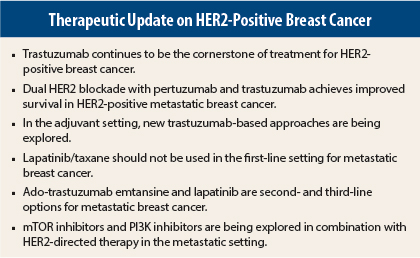
Trastuzumab (Herceptin) has been the cornerstone of therapy for HER2-positive tumors, which comprise about 20% of all breast tumors. Additional therapies targeted to other HER2 pathways or other targets to be used in combination with trastuzumab are being explored in both the adjuvant and metastatic settings. At the 2014 Chemotherapy Foundation Symposium, experts reviewed new approaches to HER2-positive breast cancer in these settings.
Adjuvant Therapy
Several drugs are now approved or under intensive study for adjuvant therapy of HER2-positive breast cancer: trastuzumab, pertuzumab (Perjeta), ado-trastuzumab emtansine (formerly known as T-DM1 [Kadcyla]), lapatinib (Tykerb), and the investigational tyrosine kinase inhibitor neratinib. These treatments act differently, explained José Baselga, MD, Physician-in-Chief at Memorial Sloan Kettering Cancer Center and President-Elect of the American Association for Cancer Research.1
“Trastuzumab has aged extremely well,” Dr. Baselga told the audience. Overall survival and disease-free survival benefits of trastuzumab are well established in the adjuvant setting. It has also been established that 2 years of trastuzumab is not better than 1 year, and 1 year should be the standard of care at present. “But we are not sure that 12 months is a magic number,” Dr. Baselga noted.
The CLEOPATRA trial showed that dual blockade of HER2 with docetaxel and pertuzumab plus trastuzumab achieved an overall survival improvement of 16 months compared with docetaxel and trastuzumab in metastatic HER2-positive breast cancer.2
“This represents great progress, and now there are studies of trastuzumab/pertuzumab in the adjuvant setting,” he said. They included the phase II NEOSPHERE trial, which showed a doubling of pathologic complete response rate with the combination of pertuzumab/trastuzumab plus docetaxel compared with trastuzumab/docetaxel (39.3% vs 21.5%).3
The randomized, double-blind, placebo-controlled, two-arm APHINITY trial is evaluating adjuvant pertuzumab plus chemotherapy plus trastuzumab vs chemotherapy with trastuzumab plus placebo for 1 year after surgery.4
Neoadjuvant trastuzumab/lapatinib has been studied in four trials, and all of them have shown an advantage for the combination, he continued. However, in the large ALTTO trial, the combination failed to improve disease-free survival in the adjuvant setting, which was disappointing, he added.5
Another approach—sequential therapy with 1 year of trastuzumab followed by 1 year of neratinib—appears to be effective in the extended adjuvant setting, according to preliminary data from the ExteNET study showing improved disease-free survival.6 “We look forward to hearing more about this sequential therapy,” Dr. Baselga said.
Moving on, he noted, “There is tremendous excitement about [ado-trastuzumab emtansine] in the adjuvant setting.”
The phase III KATHERINE study is comparing 14 cycles of adjuvant ado-trastuzumab emtansine vs 14 cycles of trastuzumab, and 50% of planned enrollment is completed. The primary endpoint is disease-free survival.7
KAITLIN, the phase III registration trial for ado-trastuzumab emtansine, will randomize patients (after surgery and anthracycline-based chemotherapy) to ado-trastuzumab emtansine plus pertuzumab vs trastuzumab, pertuzumab, and taxane chemotherapy.8 The study is designed to determine how much chemotherapy is needed, Dr. Baselga said.
Advanced HER2-Positive Breast Cancer
Kimberly Blackwell, MD, Duke Cancer Institute, Durham, North Carolina, brought attendees up to date on targeting HER2-positive advanced breast cancer for first-line treatment (de novo or metastatic tumor recurrence) and beyond the first-line setting.9
“The introduction of trastuzumab is one of the most important breakthroughs in the treatment of breast cancer with its improvement in overall survival. This survival bar has now been raised to over 15 months with the combination of pertuzumab plus trastuzumab,” she said.
“One important study showed us what not to do for first-line metastatic breast cancer,” she continued. The MA-31 trial compared lapatinib/taxane followed by lapatinib vs trastuzumab/taxane followed by trastuzumab in 600 patients and found a progression-free survival detriment for lapatinib/taxane at the interim analysis.10 Moreover, the combination was associated with increased febrile neutropenia and grade 3 or higher diarrhea.
“We need to remember these data [ie, negative findings for lapatinib/taxane] in clinical decision-making when lining up our therapeutic options for women facing this disease,” she emphasized.
Another very important study, CLEOPATRA,2 showed the power of adding a second antibody to trastuzumab as first-line treatment in the metastatic setting [see above]. Final results showed improved progression-free and overall survival with pertuzumab/trastuzumab.
“CLEOPATRA showed that it is important to first give six cycles of docetaxel [with antibody],” she said. “Getting the chemotherapy out of the way allows the targeted agents to work in this scenario without the chemotherapy toxicity.”
“With limited toxicity, pertuzumab/trastuzumab has become the preferred treatment regimen in patients diagnosed with de novo advanced or recurrent HER2-positive breast cancer,” Dr. Blackwell stated.
Second- and Third-Line Options
Once patients progress on this regimen, therapeutic options include ado-trastuzumab emtansine and lapatinib.
In the EMILIA trial, ado-trastuzumab emtansine offered significantly superior survival compared with lapatinib/capecitabine in the second- and third-line settings (P < .001), with a favorable toxicity profile.11 When EMILIA was designed, the standard of care was lapatinib/capecitabine, Dr. Blackwell explained. Ado-trastuzumab emtansine achieved a median overall survival of 30.9 months vs 25.1 months with lapatinib/capecitabine (P = .0007).
“This survival benefit is similar to what we saw initially with trastuzumab. This study, plus CLEOPATRA, show how far we have come,” she stated.
Ado-trastuzumab emtansine has different toxicities than chemotherapy, she said, mainly elevated liver enzymes and grade 3 and 4 cytopenias.
Based on the TH3RESA trial, ado-trastuzumab emtansine is a reasonable choice for third-line therapy and beyond.12 In this study, patients with at least two prior lines of HER2-directed therapy were randomized to receive ado-trastuzumab emtansine or physician’s choice of chemotherapy. Ado-trastuzumab emtansine produced a 2.9-month advantage in progression-free survival, and overall survival has not yet been reached.
A phase III study provided additional support for dual HER2 blockade, this time with lapatinib/trastuzumab vs lapatinib alone, showing that patients with HER2-driven metastatic breast cancer derived a survival benefit from HER2 combination therapy.13 The median overall survival was 51 weeks for the combination vs 39 months for lapatinib alone.
“Choice of third-line therapy should be based on patient characteristics and toxicity,” Dr. Blackwell said.
“Several ongoing trials will tell us about new options once trastuzumab, pertuzumab, [ado-trastuzumab emtansine], and lapatinib have been optimally utilized. Promising approaches include mTOR inhibitors [everolimus (Afinitor), the MARIANNE, BOLERO 1, and BOLERO 3 trials], and PI3 kinase inhibitors,” she said. ■
Disclosure: Drs. Baselga and Blackwell reported no potential conflicts of interest.
References
1. Baselga J: Adjuvant therapy for HER2-positive breast cancer. 2014 Chemotherapy Foundation Symposium. Presented November 6, 2014.
2. Swain SM, Kim SB, Cortés J, et al: Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 14:461-471, 2013.
3. Gianni L, Pienkowski T, Im YH, et al: Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (neoSphere): A randomised, open-label, phase 2 trial. Lancet Oncol 13:25-32, 2012.
4. U.S. National Institutes of Health: A study of pertuzumab in addition to chemotherapy and Herceptin (trastuzumab) as adjuvant therapy in patients with HER2-positive primary breast cancer. Available at ClinicalTrials.gov/show/NCT01358877. Accessed November 17, 2014.
5. Piccart-Gebhart MJ, Holmes MP, Baselga J, et al: First results from the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone, trastuzumab alone, their sequence, or their combination in the adjuvant treatment of HER2-positive early breast cancer. 2014 ASCO Annual Meeting. Abstract LBA4. Presented June 1, 2014.
6. U.S. National Institutes of Health: Study evaluating the effects of neratinib after adjuvant trastuzumab in women with early stage breast cancer (ExteNET). Available at ClinicalTrials.gov/show/NCT00878709. Accessed November 17, 2014.
7. U.S. National Institutes of Health: A study of trastuzumab emtansine versus trastuzumab as adjuvant therapy in patients with HER2-positive breast cancer who have residual tumor in the breast or axillary lymph nodes following preoperative therapy (KATHERINE). Available at ClinicalTrials.gov/show/NCT01772472. Accessed November 17, 2014.
8. U.S. National Institutes of Health: A study of Kadcyla (trastuzumab emtansine) plus Perjeta (pertuzumab) following anthracyclines in comparison with Herceptin (trastuzumab) plus Perjeta and a taxane following anthracyclines as adjuvant therapy in patients with operable HER2-positive primary breast cancer. Available at ClinicalTrials.gov/show/NCT01966471. Accessed November 17, 2014.
9. Blackwell K: New agents for advanced HER2-positive breast cancer. Chemotherapy Foundation Symposium. Presented November 6, 2014.
10. Gelmon KA, Boyle F, Kaufman B, et al: Open-label phase III randomized controlled trial comparing taxane-based chemotherapy with lapatinib or trastuzumab as first-line therapy for women with HER2-positive metastatic breast cancer: Interim analysis of NCIC CTG MA.31/GSK EGF 108919. 2012 ASCO Annual Meeting. Abstract LBA671.
11. Verma S, Miles D, Gianni L, et al: Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783-1791, 2012.
12. Krop IE, Kim SB, González-Martin A, et al: Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol 15:689-699, 2014.
13. Blackwell KL, Burstein HJ, Storniolo AM, et al: Overall survival benefit with lapatinib in combination with trastuzumab for human epidermal growth factor receptor 2-positive metastatic breast cancer: Final results from the EGF104900 study. J Clin Oncol 30:2585-2592, 2012.