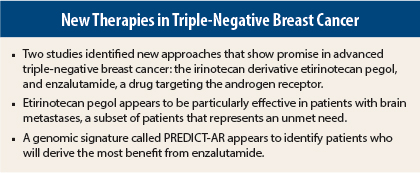
Two agents targeting novel pathways show promise in metastatic triple-negative breast cancer, according to separate studies presented at the 2015 ASCO Annual Meeting and reviewed at the Best of ASCO® meeting by Steven J. Isakoff, MD, PhD, of Massachusetts General Hospital, Boston.
The first study suggested that an irinotecan cousin, etirinotecan pegol (NKTR-102), may provide clinically meaningful benefit to patients with late-stage triple-negative breast cancer, especially those with brain metastases, which represents an unmet need.1
The second study revealed that the androgen receptor—a major target in prostate cancer—is also a valid therapeutic target in androgen receptor–positive triple-negative breast cancer,2 which accounts for 10% to 50% of all triple-negative breast cancers. Enzalutamide (Xtandi) achieved clinical benefit in patients who expressed the androgen receptor and would otherwise have been slated for more toxic chemotherapy.
Etirinotecan Pegol
The phase III BEACON study, authored by Edith A. Perez, MD, of the Mayo Clinic, Jacksonville, and colleagues, showed promising results for etirinotecan pegol in a cohort of patients with triple-negative breast cancer.
BEACON was designed to compare etirinotecan pegol vs physician’s choice (any of the following agents: eribulin, vinorelbine, gemcitabine, taxane, or ixabepilone) in 852 patients with disease progression following anthracycline, taxane, and capecitabine. About 25% of patients had triple-negative breast cancer.
At baseline, about 8% of patients in both groups had stable brain metastases, and 53% had liver metastases. Lung metastases were present in 36% of the etirinotecan pegol group and 40% of the physician’s choice group.
Unfortunately, the study did not meet its primary endpoint. There was about a 2-month improvement in overall survival with etirinotecan pegol, but it was not statistically significant.
However, in a prespecified subset of 67 patients with brain metastases, etirinotecan pegol improved median overall survival by 5.1 months compared with physician’s choice: 10 vs 4.8 months (P < .01). The 1-year survival rate in patients with brain metastasis was 44.4% for etirinotecan pegol vs 19.4% for physician’s choice.
Similarly, in 456 patients with liver metastases, median overall survival was significantly improved with etirinotecan pegol: 10.9 vs 8.3 months, respectively (P = .002).
Etirinotecan pegol had lower rates of toxicity compared with physician’s choice, with very few grade 3 or higher toxicities. Biomarker studies to identify predictors of response and outcomes are underway.
“Where this drug gets interesting is a provocative finding of doubling of survival in the subset of patients with brain metastases, which represents only 67 patients in this study. Improved survival in patients with liver metastases is also of interest. Etirinotecan pegol also significantly improved global health on the EORTC [European Organisation for Research and Treatment of Cancer] Quality of Life [questionnaire],” Dr. Isakoff said.
“Overall, etirinotecan pegol has clinical activity and good tolerability in heavily pretreated patients, but survival is not statistically significantly improved in advanced breast cancer. The significant improvements in outcome in the groups with brain metastases and liver metastases deserve further study,” he said.
Dr. Isakoff pointed out that there are at least three molecules with an irinotecan molecule backbone being studied in breast cancer: etirinotecan pegol, an antibody drug conjugate called IMMU-132, and MM-398.
Enzalutamide
A phase II study, presented at the 2015 ASCO Annual Meeting by Tiffany A. Traina, MD, of Memorial Sloan Kettering Cancer Center, New York, suggested that enzalutamide—a drug currently approved for treatment of metastatic castration-resistant prostate cancer—may hold promise for the treatment of androgen receptor–positive triple-negative breast cancer. This is the first report to show clinical responses in breast cancer.
The androgen receptor is expressed in 10% to 50% of patients with triple-negative breast cancer, Dr. Isakoff said. For enrollment in this study, androgen-receptor positivity was considered to be any expression greater than 0% by immunohistochemistry. Of 165 patients screened, 118 had some degree of androgen-receptor expression. Evaluable patients were those with androgen-receptor expression > 10% on immunohistochemistry, which represented 55% of the study population.
The primary endpoint was clinical benefit (defined as complete or partial response or stable disease) at 16 weeks. Among evaluable patients, the clinical benefit ratio was 35% at 16 weeks. In the intent-to-treat population, the clinical benefit rate was 25% at 16 weeks.
Among evaluable patients, there were two complete responses and five partial responses. “This is fascinating. There are RECIST [Response Evaluation Criteria in Solid Tumors] responses to androgen blockade, which has never been reported before,” Dr. Isakoff stated.
Median progression-free survival was 14 weeks. In the intent-to-treat population, higher androgen-receptor expression was associated with longer progression-free survival: Progression-free survival for androgen-receptor expression > 10% vs < 10% was 14 vs 8 weeks, respectively.
Dr. Traina and colleagues analyzed 404 tissue samples with gene profiling and came up with an androgen receptor–related gene signature for androgen-receptor positivity called PREDICT-AR. This signature was found to predict clinical benefit and may be useful for future studies of androgen blockade in breast cancer, Dr. Isakoff said.
Patients whose tumors expressed the signature had a 16-week clinical benefit rate of 40% vs 11% for those whose tumors did not express the signature. PREDICT-AR also predicted improvement in progression-free survival: Those who tested positive for PREDICT-AR had a median progression-free survival of 16 weeks vs 8 weeks in those who tested negative for PREDICT-AR.
Among evaluable patients who were PREDICT-AR–positive and treated in the first or second line, median progression-free survival was 40+ weeks, Dr. Traina told The ASCO Post. “That’s remarkable in triple-negative breast cancer,” she commented.
“It is clear that enzalutamide has clinical activity in androgen receptor–positive triple-negative breast cancer, with consistent safety. The novel diagnostic assay, PREDICT-AR, will be studied further,” Dr. Isakoff said.
“It is important to note that triple-negative breast cancer is heterogeneous—it is an immunohistochemical basket with at least five different subtypes amenable to different treatments. Androgen receptor is clearly expressed in a substantial proportion of patients—at least 50% in this study.”
“The PREDICT-AR signature is also common, found in about 50% of advanced triple-negative breast cancers, and is worthy of further development. It is encouraging that we are learning how to predict response,” Dr. Isakoff said. ■
Disclosure: Drs. Isakoff, Perez, and Traina reported no potential conflicts of interest.
References
1. Perez EA, Awada A, O’Shaughnessy J, et al: ASCO Annual Meeting. Abstract 1001. Presented June 1, 2015.
2. Traina TA, Miller K, Yardley DA, et al: ASCO Annual Meeting. Abstract 1003. Presented June 1, 2015.