In a study exploring the effect of primary debulking surgery in women with bulky stage IIIC ovarian, fallopian tube, and primary peritoneal cancers, cytoreduction to no gross residual disease was associated with the best survival outcomes.1 Cytoreduction to 1 to 10 mm of residual disease was also associated with survival benefit as compared to residual disease greater than 10 mm.
“Although primary debulking surgery to no gross residual disease was associated with the longest progression-free survival and overall survival, cytoreduction to 1 to 10 mm of residual disease was also associated with significantly prolonged progression-free survival and overall survival compared with more than 10 mm of residual disease,” said Vasileios Sioulas, MD, Fellow in Gynecologic Oncology at Memorial Sloan Kettering Cancer Center, New York.
Despite the lower survival rates for patients with more than 10 mm of residual disease, Dr. Sioulas noted that they were “very similar” to those reported in the literature for patients treated with neoadjuvant chemotherapy.
“The administration of neoadjuvant chemotherapy should not automatically be the option chosen for patients who cannot undergo primary cytoreduction to no gross residual disease,” he said, “especially if intraperitoneal chemotherapy can be given after the primary debulking surgery.”
As Dr. Sioulas reported at the 2016 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer, primary debulking surgery followed by platinum- and taxane-based chemotherapy is a cornerstone of management of advanced ovarian cancer. However, two recently published, randomized, controlled trials have found the use of platinum-based primary chemotherapy followed by delayed surgery to be an effective and safe alternative treatment regimen.2,3 In addition, it has been suggested that neoadjuvant chemotherapy and interval debulking surgery to allow for no gross residual disease might be superior to primary debulking surgery after which the patient is left with gross residual disease.
Study Design and Results
Dennis Chi, MD, Head of Ovarian Cancer Surgery at Memorial Sloan Kettering Cancer Center, along with Dr. Sioulas, and colleagues retrospectively reviewed 496 patients with stage IIIC ovarian, fallopian tube, or primary peritoneal carcinoma who underwent primary debulking surgery at Memorial Sloan Kettering between January 2001 and December 2010. Patients with borderline tumors or nonepithelial histology were excluded from the study. The median follow-up was 53 months, and the median age of the study population at the time of primary debulking surgery was 62 years.
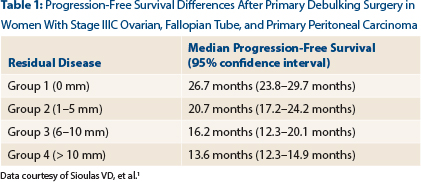
The greatest diameter of the residual tumor was noted at the end of the surgery and obtained from these respective reports. According to the reported residual disease, participants were classified into four groups: group 1 (0 mm); group 2 (1–5 mm); group 3 (6–10 mm); group 4 (> 10 mm).
As Dr. Sioulas reported, no gross residual disease at the end of primary debulking surgery was achieved in 37% of patients, and 26% of study participants were suboptimally debulked. Significant differences in progression-free survival were observed among the four patient groups, said Dr. Sioulas (Table 1). The median progression-free survival for the entire cohort was 18.6 months (95% confidence interval [CI] = 16.7–20.4 months).
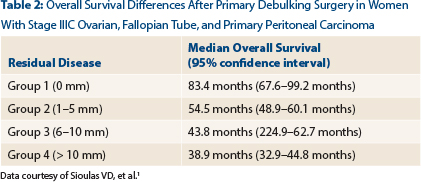
Dr. Sioulas also noted statistically significant differences between patient groups with respect to overall survival (Table 2). The median overall survival for the entire cohort was 54.7 months (95% CI = 50.8–58.7 months).
Given the relatively small number of patients with residual disease measuring 6 to 10 mm and to be consistent with the cutoff of 10 mm that is used in the vast majority of the existing literature to define optimal residual disease, patients with residual disease of 1 to 5 mm and 6 to 10 mm were combined into one group for the remaining analysis. The median overall survival for this combined group was 52.6 months.
“Importantly,” said Dr. Sioulas, “patients with residual disease between 1 and 10 mm had significantly better overall survival compared to those with residual disease greater than 10 mm [38.9 months].”
Role of Intraperitoneal Chemotherapy
In addition, 42% of patients with either no gross residual disease or residual disease between 1 and 10 mm at the end of the primary debulking surgery received at least one cycle of primary intravenous/intraperitoneal chemotherapy.
Managing Stage IIIC Gynecologic Cancer
Primary debulking surgery to no gross residual disease was associated with the best survival outcomes in patients with bulky stage IIIC ovarian, fallopian tube, and primary peritoneal carcinoma. Primary debulking surgery to 1 to 10 mm residual disease was associated with significantly better survival outcomes, compared to > 10 mm residual disease. The addition of intraperitoneal chemotherapy to the primary treatment regimen was associated with improved overall survival in patients with residual disease between 1 and 10 mm.“Similar to the results of the retrospective analysis of the GOG 114 and 172 studies, the addition of intraperitoneal chemotherapy to the primary treatment regimen was associated with significantly improved overall survival in patients with residual disease between 1 and 10 mm,” Dr. Sioulas reported (65.1 vs 40.6 months). Finally, the survival outcomes of the suboptimally debulked patients with greater than 10 mm of residual disease were comparable to those treated with neoadjuvant chemotherapy, as reported in the EORTC 55971 and CHORUS trials.
“Taken together, if primary debulking surgery to no gross residual disease is not feasible, the administration of neoadjuvant chemotherapy should not automatically be the chosen option, especially if intraperitoneal chemotherapy can be given after cytoreduction to residual disease of 1 to 10 mm,” Dr. Sioulas concluded. ■
Disclosure: Dr. Sioulas reported no potential conflicts of interest.
References


