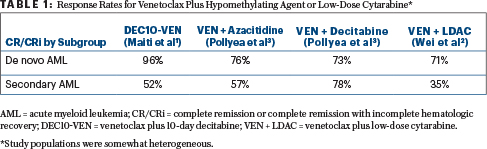
The benefit of adding venetoclax to a hypomethylating agent or low-dose cytarabine in the front-line treatment of acute myeloid leukemia (AML) was evident from a number of studies reported at the 2018 American Society of Hematology (ASH) Annual Meeting & Exposition (see Table 1). For elderly patients or those who are unfit for intensive chemotherapy, venetoclax plus azacitidine or decitadine or low-dose cytarabine recently received accelerated approval, with full approval contingent on confirmatory data.
Venetoclax Plus 10-Day Decitabine
In a phase II study from The University of Texas MD Anderson Cancer Center, venetoclax plus 10-day decitabine resulted in very high response rates in the front-line and salvage settings, according to Abhishek Maiti, MBBS, of The University of Texas MD Anderson Cancer Center, Houston.1 The study evaluated decitabine at 20 mg/m2 for 10 days per cycle (rather than the established 5 days) as induction therapy, as this schedule has been shown to improve response rates in both newly diagnosed and relapsed patients, he explained.

Abhishek Maiti, MBBS
In an ongoing study, an interim analysis of the first 48 patients (median age of 71) was presented. The population included patients who had newly diagnosed AML or untreated secondary AML as well as those who had relapsed or refractory AML or myelodysplastic syndromes (MDS) and previously treated secondary AML.
Venetoclax was given on days 1 to 28 in cycle 1 and days 1 to 21 in cycle 2 onward; however, dosing was interrupted on cycle 1, day 21 if the 21-day bone marrow showed clearance of blasts, until count recovery. Venetoclax was dosed at 200 mg daily in combination with CYP3A4 inhibitors such as “azole” antifungal therapy, and decitabine was given at 20 mg/m2 for 10 days every 4 to 8 weeks for induction, followed by decitabine for 5 days after achievement of response. Treatment was discontinued upon progressive disease or lack of response after four cycles. The study allowed the concomitant use of approved tyrosine kinase inhibitors (FLT3 or BCR-ABL kinase inhibitors) as appropriate.
After a median follow-up of 5.2 months and a median of 3 treatment cycles, among newly diagnosed patients, 96% achieved complete remission (59%) or complete remission with incomplete hematologic recovery (37%), with a median time to response of 42 days. The percentage of patients who tested negative for minimal residual disease (MRD) in this cohort was 52%, and there were no deaths within 30 and 60 days, Dr. Maiti reported.
In patients with relapsed or refractory disease and in those who had prior treatment for secondary AML, the rate of complete remission or complete remission with incomplete hematologic recovery was 52%, with 50% of patients achieving MRD negativity. The median time to response was 76 days, and in this sicker group of patients, the mortality rate was 19% at 30 days and 24% at 60 days.
“Among 13 patients with AML who were refractory to prior hypomethylating agent therapy, the complete remission or complete remission with incomplete hematologic recovery rate was 75%. The median duration of response was 1.1 months (range, 0.5 to not reached). Thus, even though patients with relapsed AML can obtain a remission with this strategy, duration is often short, and transplantation should be considered if possible,” he commented.
In total, there were 16 deaths, the majority in the relapsed or refractory cohort; most of them were from infections (9 patients). There were no early deaths in newly diagnosed patients. Grade 3 to 4 neutropenia occurred in 46% of patients, and 3% were grade 5. Grade 3 to 4 febrile neutropenia was seen in 15%, and 2% had grade 3 to 4 tumor-lysis syndrome. “Toxicities were manageable,” according to Dr. Maiti.
Interestingly, the investigators saw high response rates in newly diagnosed and secondary untreated patients with AML who had relevant mutations: 100% in those with IDH1/2, NPM1,ASXL1, and RUNX1 mutations; 75% in those with FLT3 and RAS mutations. The response rate was 100% in 4 patients with TP53 mutation.
At 6 months, the overall survival rate was 85% in the newly diagnosed and secondary untreated AML group and 52% in the relapsed or refractory and treated secondary AML group; disease-free survival was 82% and 55%, respectively.
Venetoclax Plus Low-Dose Cytarabine
Venetoclax was paired with low-dose cytarabine in a phase I/II study of previously untreated patients with AML who were ineligible for intensive chemotherapy due to age or comorbidities.2 “Venetoclax plus low-dose cytarabine produced rapid and durable remissions in previously untreated patients with AML who are ineligible for intensive chemotherapy, demonstrating a tolerable safety profile,” said Andrew Wei, MBBS, PhD, of The Alfred Hospital and Monash University in Melbourne, Australia. “This is an attractive treatment option for these patients.”

Venetoclax plus low-dose cytarabine produced rapid and durable remissions in previously untreated patients with AML who are ineligible for intensive chemotherapy.— Andrew Wei, MBBS, PhD
Tweet this quote
The study included 82 patients 60 years of age or older (median age was 74) deemed unfit for intensive chemotherapy, including patients with previously untreated AML and those with secondary AML. This study differs from some others in that patients could have received a hypomethylating agent for preexisting myeloid disorders. Almost half the study population had secondary AML, and 30% had received prior hypomethylating agents for MDS before enrollment to the trial.
In the initial cohort, low-dose cytarabine (20 mg/m2/d) was initiated first, on day 1. Venetoclax was added on day 2 and ramped up over 5 days, to a target dose of 600 mg, and then patients were continued on that dose. In the expansion cohort, low-dose cytarabine and venetoclax were initiated concurrently, with venetoclax started at 100 mg and escalated to 600 mg over 4 days. One patient in each cohort showed laboratory evidence of tumor-lysis syndrome, without clinically significant features. The use of CYP3A4 inhibitors was allowed with appropriate venetoclax dose reductions.
The complete remission or complete remission with incomplete hematologic recovery rate was 54% overall (26% complete remission). By key subgroups, complete remission or complete remission with incomplete hematologic recovery rates were 71% for patients with de novo AML; 35% for patients with secondary AML; 62% for patients without prior hypomethylating agent exposure; 33% for those who had prior hypomethylating agent treatment; 63% for patients with a intermediate-risk cytogenetics; and 42% for those with poor-risk cytogenetic features. The median duration of remission was 8.1 months. MRD negativity was seen in 32% of patients who achieved complete remission or complete remission with incomplete hematologic recovery.
“Many responses were seen quickly,” Dr. Wei noted. “Typically, when we think about low-intensity therapy, like low-dose cytarabine alone, we expect a response rate of less than 20%. With a hypomethylating agent by itself, we may need to wait 4 to 6 months to see the peak effect. Here, the median time to first response was 1.4 months and to best response, 2.8 months. Consequently, patients are receiving the benefits of being in remission much more rapidly than before.”
The complete remission or complete remission with incomplete hematologic recovery rates varied widely by mutation status: NPM1 (89%), IDH1/2 (72%), FLT3 (44%), and TP53 (30%). Transfusion independence was achieved in 60% of patients requiring platelets, 48% requiring red blood cells, and 46% requiring both, lasting more than 200 days.
The median overall survival was 10.1 months, and the 30-day mortality rate was 6%. By response category, the median overall survival was not reached for patients achieving a complete remission and was 18.4 months for the complete remission or complete remission with incomplete hematologic recovery subgroup, but it fell to 3.5 months for patients who did not achieve a response. At 12 months, 100% of patients achieving a complete remission were alive, vs 73% of the complete remission or complete remission with incomplete hematologic recovery subgroup and 5% for patients not achieving these endpoints.
“This suggests survival is driven by response to the protocol--defined therapy, as opposed to the subsequent therapies that may have benefited these patients,” he said.
Treatment-emergent and serious adverse events were mostly those characteristic of other AML therapies, he reported.
Venetoclax Plus Hypomethylating Agent
Daniel A. Pollyea, MD, of the University of Colorado, Denver, presented findings from a phase Ib expansion trial of venetoclax in combination with decitabine or azacitidine in 115 patients with untreated de novo AML or secondary AML who were ineligible for intensive chemotherapy.3 Patients received 400 mg daily in a 3-day dose ramp-up coadministered with either decitabine at 20 mg/m2 on days 1 to 5 (n = 31; median age, 72) or azacitidine at 75 mg/m2 on days 1 to 7 (n = 84; median age, 75) within each 28-day cycle. Dose adjustments for venetoclax were implemented as appropriate.

These results suggest that venetoclax, in combination with hypomethylating agents, may provide a potent therapeutic option for patients with AML who are not eligible for intensive chemotherapy.— Daniel A. Pollyea, MD
Tweet this quote
“Deep and durable responses were observed. The majority of those responses were complete remissions,” Dr. Pollyea reported. The complete remission or complete remission with incomplete hematologic recovery rates were 71% for venetoclax/azacitidine and 74% for venetoclax/decitabine, and these rates were largely irrespective of baseline genetic mutations and cytogenetic risk. There were also not many discernible differences for de novo and secondary AML.
“And as with venetoclax plus 10-day decitabine or low-dose cytarabine, there was a propensity for good responses in patients with IDH1/2-mutated and NPM1-mutated disease,” he added.
The median follow-up was 14.9 months for the azacitidine group and 16.2 months for the decitabine arm. At 12 months, 69% and 57% of patients, respectively, were continuing to respond to treatment. Early mortality was observed in 2% and 7%, respectively.
The median overall survival was 16.9 months for venetoclax/azacitidine and 16.2 months for venetoclax/decitabine. More than half the patients who required transfusions at baseline became transfusion-independent. Across both treatment groups, among patients with complete remission or complete remission with incomplete hematologic recovery, 45% achieved MRD negativity. Patients who received a venetoclax dose reduction for CYP3A inhibitors had similar responses as those who did not. No unexpected adverse events were observed.
Venetoclax in AML
- Venetoclax was paired with hypomethylating agents or low-dose cytarabine in several studies presented at the 2018 ASH Annual Meeting & Exposition.
- In newly diagnosed patients with AML, the rate of complete remission/complete remission with incomplete recovery ranged from 54% to 96%.
- In patients with relapsed or refractory disease, complete remission or complete remission with incomplete hematologic recovery rates ranged from 35% to 78%.
“These results suggest that venetoclax, in combination with hypomethylating agents, may provide a potent therapeutic option for patients with AML who are not eligible for intensive chemotherapy,” Dr. Pollyea concluded. ■
DISCLOSURE: Dr. Maiti has received institutional research funding from Celgene. Dr. Wei has consulted for or received honoraria from Novartis, Amgen, Celgene, Servier, and AbbVie; and has received research funding from Celgene, Servier, and AbbVie. Dr. Pollyea has consulted or served on the advisory or director’s board for Pfizer, Celyad, Agios, Argenx, AbbVie, Karyopharm, Celgene, Gilead, and Curis; and has received research funding from Pfizer, Agios, and AbbVie.
REFERENCES
1. Maiti A, DiNardo CD, Cortes JE, et al: Interim analysis of phase II study of venetoclax with 10-day decitabine (DEC10-VEN) in acute myeloid leukemia and myelodysplastic syndrome. 2018 ASH Annual Meeting & Exposition. Abstract 286. Presented December 2, 2018.
2. Wei A, Strickland SA, Hou JZ, et al: Venetoclax with low-dose cytarabine induces rapid, deep, and durable responses in previously untreated older adults with AML ineligible for intensive chemotherapy. 2018 ASH Annual Meeting & Exposition. Abstract 284. Presented December 2, 2018.
3. Pollyea DA, Pratz KW, Jonas BA, et al: Venetoclax in combination with hypomethylating agents induces rapid, deep, and durable responses in patients with AML ineligible for intensive therapy. 2018 ASH Annual Meeting & Exposition. Abstract 285. Presented December 2, 2018.


